Introduction
Over the past decade, advancements in cellular and molecular biotechnology have facilitated the introduction of ATMPs into the EU market. To date, 26 ATMPs have received EMA approval, including 12 GTPs, 5 SCPs, 3 TEPs and 6 CAR-T therapies1. These therapies have demonstrated therapeutic benefits across various areas of medicine, such as orthopaedic diseases, neurodegenerative disorders, cancer, autoimmune and inflammatory conditions, showcasing the transformative potential of ATMPs in modern medicine. Recent safety signals have introduced critical considerations regarding patient access to ATMPs, marking a significant moment in the landscape of medical innovation and regulation. As these signals emerge, questions arise concerning the future availability and utilisation of ATMPs.
What is a safety signal?
A safety signal refers to information indicating a potential adverse event associated with a medicine, whether it’s a new finding or a known issue that requires further investigation. These signals can arise from various sources, including spontaneous reports, clinical studies, and scientific literature. They serve as early indicators of potential safety concerns and prompt regulatory agencies and healthcare professionals to conduct further assessments to determine the nature and extent of the risk posed by the medicine2.
The inherent unmet medical need and scientific uncertainties surrounding ATMPs prompt ethical considerations about striking a balance between patient needs, benefits, and safety. With limited clinical data stemming from small sample sizes in randomised controlled trials, and a dearth of long-term effectiveness data, safety signals may only be observed after regulatory approval for some ATMPs3.
Chimeric antigen receptor T-cell therapies
In late November 2023, the FDA initiated an investigation into the “serious risk” of patients developing new cancers following CAR-T therapies for blood cancers.
The FDA’s investigation has prompted regulatory action, leading to a mandate that manufacturers of certain CAR-T therapies update their labels with a black box warning. This warning states, “T cell malignancies have occurred following treatment with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including [name of drug].” Products affected by this mandate include:
- Abecma
- Breyanzi
- Carvykti
- Kymriah
- Yescarta
Tecartus, must also update its safety information, but with a revised warning which clarifies that patients treated with Tectartus specifically have not experienced T-cell malignancies4.
Although the overall benefits of CAR-T therapies continue to outweigh their potential risks, the FDA will move forward in their investigation and will also consider other serious outcomes including hospitalisation and death. The agency is currently evaluating the need for further regulatory action5.
The case of Zynteglo
Zynteglo (betibeglogene autotemcel), authorised in May 2019 for the curative treatment of transfusion-dependent β-thalassemia (TDT), encountered safety concerns regarding hematologic malignancies.
In February 2021, the European Commission (EC) began reviewing Zynteglo due to a case of acute myeloid leukaemia (AML) in a sickle-cell disease patient who received LentiGlobin, a similar product using the same lentiviral vector. The Committee for Medicinal Products for Human Use (CHMP) was tasked with assessing the risk-benefit profile of Zynteglo. Expert evaluations, supported by the Pharmacovigilance Risk Assessment Committee (PRAC) and the CAT, suggested that the lentiviral vector was unlikely to be the primary cause of the malignancies. Despite these findings, the EC decided to withdraw the marketing authorisation for Zynteglo in the EU on March 24, 2022, following a request from the Marketing Authorisation Holder (MAH). The decision to discontinue Zynteglo’s commercialisation was influenced by various factors, including manufacturing issues and failed price negotiations with health authorities6.
Impact on patient access
As ATMPs aim to target broader patient populations and earlier lines of treatment, payers may resist funding, and patient access may become more difficult. HTA and regulatory bodies, which already exercise caution when evaluating ATMPs, may heighten their scrutiny7. For example, in January 2024, the EMA announced the initiation of a review by its PRAC regarding secondary cancers associated with CAR-T therapies. The review specifically focuses on data from 23 cases involving T-cell lymphoma or leukaemia8. Figure 1 highlights the possible impact of ATMP safety signals on patient access.
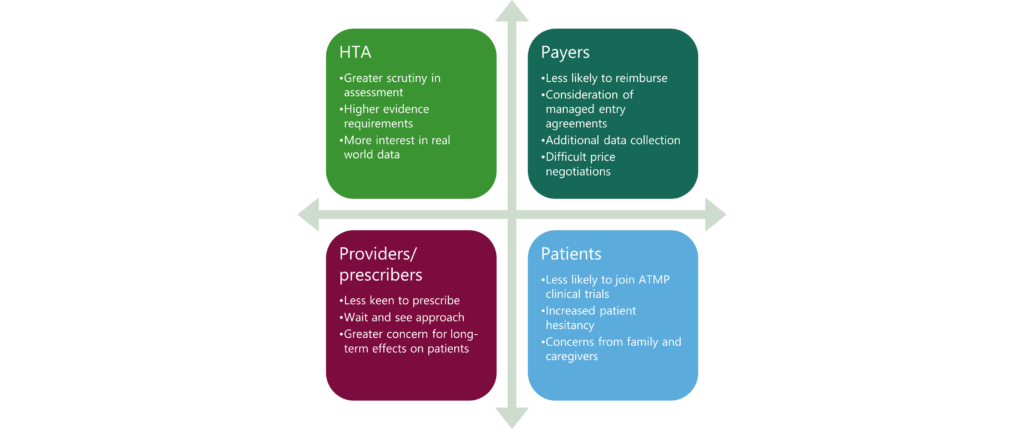
Despite these concerns, the risk-benefit assessment of ATMPs remains positive and most challenges are driven by commercial factors. Challenges such as poor commercial performance, lack of reimbursement, and variations in market access routes across different countries present significant hurdles for manufacturers, especially smaller to mid-sized companies. Moreover, the high costs of manufacturing, challenges in scaling up production, and intricate procedures contribute to elevated prices9.
Conclusion
As safety signals continue to emerge, navigating the delicate balance between innovation, safety, and patient access remains a pressing challenge in the realm of ATMPs. Collaborative efforts amongst regulatory bodies, manufacturers, healthcare providers, and patients are imperative to address safety concerns whilst ensuring equitable access to transformative therapies.
We recently attended the Advanced Therapies Congress in London, UK, and presented a piece of research on ATMP’s and their economic benefit. Explore the poster here.
Sources:
- Bellino S, La Salvia A, Cometa MF, Botta R. Cell-based medicinal products approved in the European Union: current evidence and perspectives. Front Pharmacol. 2023;14:1200808.
- Safety signal. EMA website. https://www.ema.europa.eu/en/glossary/safety-signal#:~:text=Information%20on%20a%20new%20or,found%20under%20’Signal%20management‘. Accessed March 14, 2024.
- Why is the number of advanced therapy medicinal product (ATMP) withdrawals from the European market rising? Remap Consulting website. https://remapconsulting.com/hta/why-is-the-number-of-advanced-therapy-medicinal-product-atmp-withdrawals-from-the-european-market-rising/. Accessed March 14, 2024.
- Regulatory update: FDA adds boxed warning for some CAR T-cell therapies. AABB website. https://www.aabb.org/news-resources/news/article/2024/01/24/regulatory-update–fda-adds-boxed-warning-for-some-car-t-cell-therapies. Accessed March 14, 2024.
- FDA Investigating Serious Risk of T-cell Malignancy Following BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies. FDA website. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous. Accessed March 14, 2024.
- Petitpain N, Antoine ML, Beurrier M, Micallef J, Fresse A. Pharmacovigilance of gene therapy medicinal products. Rare Disease and Orphan Drugs Journal. 2023; 2(4): 25.
- Gilead’s Tecartus dropped from the FDA’s CAR-T box warning list. https://www.linkedin.com/posts/partners4access_p4ainsights-us-fda-activity-7156275634611068928-kZHd/?utm_source=share&utm_medium=member_desktop. Accessed March 14, 2024.
- EMA Starts Safety Review of CAR T-Cell Medicines. PharmaTech website. https://www.pharmtech.com/view/ema-starts-safety-review-of-car-t-cell-medicines. Accessed March 14, 2024.
- Piemonti L, Scholz H, de Jongh D, et al. The Relevance of Advanced Therapy Medicinal Products in the Field of Transplantation and the Need for Academic Research Access: Overcoming Bottlenecks and Claiming a New Time. Transpl Int. 2023;36:11633.