Introduction
The CMS has proposed changes to CAR-T reimbursement in the US which could potentially have a negative financial impact on treatment providers. Here we discuss the proposed changes and consider the outstanding questions/implications.
Since the first FDA approval of a CAR-T treatment in 2017, concerns have persisted about how the Medicare program would cover the very high cost of these products (with an average sales price of $373,000). Hospital inpatient reimbursement is calculated on an episodic basis using a MS-DRG base payment rate, new technology add-on payment (NTAP), and outlier payments.
In FY2022, in-patient stays with CAR-T treatment are assigned to DRG018 which has a base rate of $246,955. Hospitals can currently receive additional payments, via a new technology add-on payment (NTAP), for two products (Tecartus [brexucabtagene autoleucel] and Abecma [idecabtagene vicleucel]), with the NTAP limited to 65% of the product cost. Additional outlier payments apply in instances where the costs exceed the MS-DRG payment, the NTAP amount (where applicable), and the fixed-loss threshold of $30,988. However, it has been reported that these fees sometimes do not cover the costs of CAR-T treatment.
Proposed Changes for FY2023
For FY2023, the CMS proposed several policies that would impact provider reimbursement for CAR-T (see Figure 1).
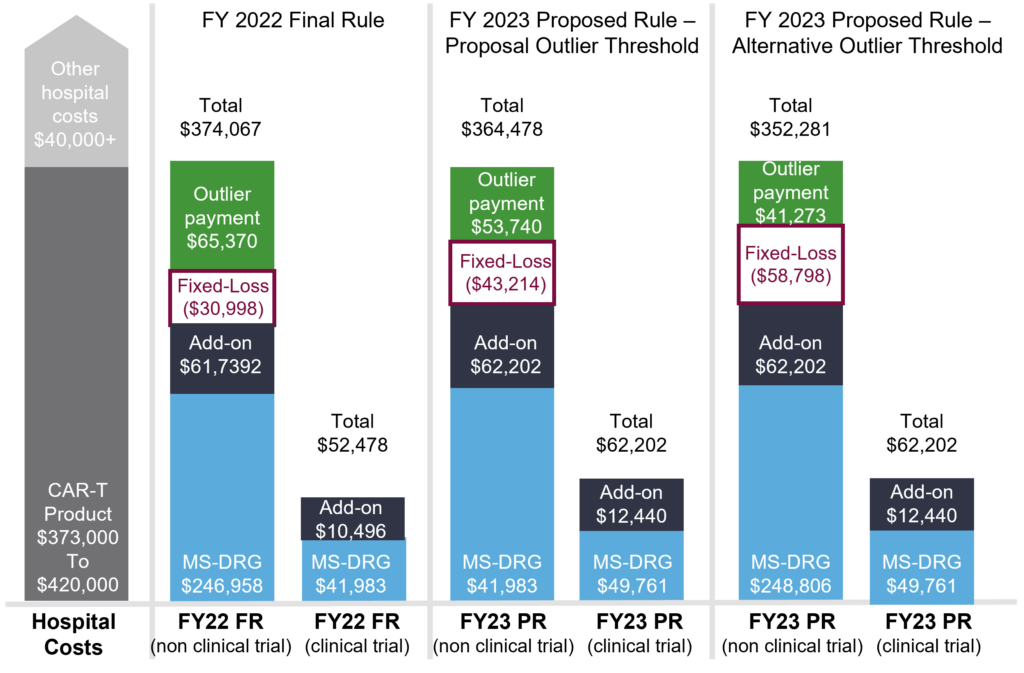
Payment Changes for CAR-T Cases
The proposed base payment for CAR-T cases in FY2023 would increase to $248,806. However, the CMS proposes to increase the fixed-loss amount (which raises the threshold for outlier payments to be issued) to $43,214, which is anticipated to lower overall reimbursement in FY2023.
Use of 2021 Data to Establish Payment
The CMS propose using 2021 Medicare Provider Analysis and Review (MedPAR) data to set relative weights for MS-DRGs resulting in the hospital charge data including the use of three additional products, Tecartus, Breyanzi (lisocabtagene maraleucel) and Abecma.
Product NTAP Decisions
Two CAR-T treatments with NTAP status in FY2022 will continue in FY2023 – Abecma and Tecartus. The CMS will accept public comment on whether Carvykti (ciltacabtagene autoleucel) meets the criteria for NTAP status in FY2023.
Outstanding Questions and Implications
There are a couple of additional considerations that may impact future reimbursement calculations for CAR-Ts, namely:
- Alternative Payment and Value-Cased Arrangements: In the near future the CMS may consider whether innovative financing approaches should extend to the Medicare fee-for-service or Medicare Advantage space.
- Potential for Shifts in Site of Care: As CAR-T treatments shift increasingly to outpatient settings, it may lead to changes to current reimbursement.
Sources
Avalere Health, “CAR-T Reimbursement Continues to Evolve in FY2023 IPPS Proposed Rule Avalere Health”, 28th April 2022