What pricing and market access processes exist to encourage patient access
Access to orphan drugs varies substantially across the EU5. Differences in national market access and reimbursement processes are an important factor in determining the extent of patient access to orphan drugs. This newsletter will discuss the variation in access to orphan drugs observed across the EU5 and mechanisms by which individual countries may encourage access.
Introduction
Orphan drugs are targeted toward treating rare diseases with smaller patient populations. Orphan designation is generally given to products targeting life-threatening or debilitating diseases where the prevalence falls below a fixed threshold (such as 5 in 10,000 in the EU) or where the profit generated by the medicine is unlikely to be sufficient to justify its research and development costs. As a result, incentives such as fee reductions, protocol assistance and ensured market exclusivity periods, are generally required to encourage the development and launch of orphan drugs.
Through these measures, access to medical interventions can be expanded to enable the treatment of a wider variety of patients, promoting more equal access to care within society. Despite the benefits associated with funding orphan drugs, small patient populations and limited clinical data often results in orphan drugs finding it challenging to demonstrate cost-effectiveness.
Ultimately, funding is a subjective decision requiring individual countries to make a judgement call, based on their own ethical and moral opinions, regarding the extent that cost-savings should be sacrificed in order to fund orphan drugs and promote more equal access to care. This leads to variation in the market access processes and requirements between countries and so variation in the uptake of orphan drugs.
Variation in access to orphan drugs across the EU5
An assessment of the variation in access within the EU5 has recently been undertaken comparing the number of orphan medicinal products (OMPs) obtaining European marketing authorisation between the year 2000 and May 2016 with the number of OMPs achieving reimbursement within the EU5 (Zamora et al., 2019).
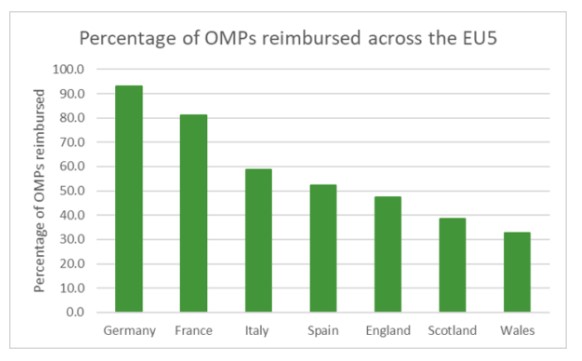
Figure 1. Percentage of OMPs reimbursed across the EU5 having gained European marketing approval between the year 2000 and May 2016. Adapted from Figure 1 in Zamora et al., 2019.
Where access is defined as the full or partial reimbursement of the OMP, Germany and France appear to demonstrate the highest levels of access within the EU5, with 93.0% and 81.1% of centrally approved OMPs showing some level of reimbursement. This is substantially higher than the levels of access observed in Italy, Spain and the UK (58.7%, 52.4% and 47.6-32.9% respectively).
Mechanisms for promoting access to orphan drugs
This variation in access is presumably due to the different market access and reimbursement processes implemented in each country. In Germany, the high levels of access observed is partly achieved through the automatic classification as possessing additional benefit during the AMNOG HTA process, providing that sales volumes do not exceed €50 million (Bouslouk, 2016).
Similarly, in France, the criteria for the assessment of improvement in actual benefit is generally favourable towards orphan drugs, which often meet expectations regarding disease severity and unmet need. In fact, between the years 2000 and 2016, 40% of orphan drugs received an ASMR rating of Ⅰ to Ⅲ, compared to only 3% for drugs of all types (Mennezein et al., 2017). These adaptations likely assist manufacturers during pricing and reimbursement negotiations.
In contrast, there are fewer opportunities to demonstrate value within the other EU5 countries, such as the UK, where special assessment criteria are not utilised to the same extent. Small modifications to assessment criteria are implemented by the SMC and AWMSG, but orphan drugs are still required to undergo a standard technology appraisal. Pathways exist for the assessment of ultra-orphan and oncology drugs, such as the Highly Specialised Technologies pathway and the Cancer Drugs Fund, but these only apply for a subset of orphan drugs.
Discussion
Enabling orphan drugs to demonstrate value within reimbursement systems is essential to facilitate the treatment of rare diseases. However, the value of rarity and the best way to assess it remain important questions for payers to consider. Improvements in precision medicine approaches to treatment have the potential to stratify existing diseases into smaller sub-disease patient populations. This could necessitate the use of multiple orphan drugs in place of broader methods of treatment in order to allow the best possible standard of care and promote improved patient outcomes. This would undoubtably put strain on healthcare budgets, with the high price of many recently launched orphan drugs being a matter of concern for many. As a result, innovative pricing solutions will be required to reimburse based on their true value to payers and enable their access. This is needed to promote the best outcomes for patients while reducing the sacrifices in overall population health necessary for the commissioning of orphan drugs.
References:
Bouslouk, M. 2016. G-BA benefit assessment of new orphan drugs in Germany: the first five years. Expert Opinion on Orphan Drugs, 4(5), 453-455.
Mennezein, L., Avot, D and Laigle, V. 2017. Orphan Drugs In France: Key Market Access Incentives. Value in Health, 20(9), A565.
Zamora, B., Maignen, F., O’Neill, F., Mestre-Ferrandiz, J and Garau M. 2019 Comparing access to orphan medicinal products in Europe. Orphanet Journal of Rare Diseases, 14(95).
