Introduction
In early 2022, the EU HTA regulation was formally ratified with the aim of establishing an EU-wide joint assessment of clinical effectiveness with an accompanying process for joint early scientific dialogue with EU HTA agencies. With the first products anticipated to go through this process in 2025, we discuss the developments that have been made to the process in 2022, linking them back to predictions we made at the beginning of the year, and looking at what we can expect in 2023.
Key changes in 2022
At the beginning of the year, we made three predictions regarding the implementation of EU HTA. Overall, these three predictions have all come to fruition.
Prediction One – EU HTA regulation to come into force in early 2022
Our prediction: The European Parliament plenary vote will likely take place in December and the EU HTA Regulation will likely come into force by Q1 2022.
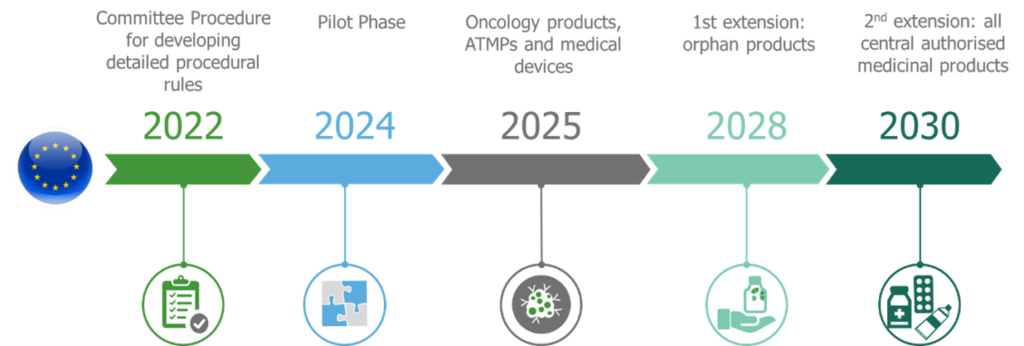
What happened: In January 2022, Regulation 2021/2282, which amended Directive 2011/24/EU on cross-border health care, came into force after it was published in the Official Journal of the European Union in December 2021. The most important implication of the regulation is the introduction of EU-level joint clinical assessments (JCAs) that will become applicable in a few years’ time.
Its provision relating to JCAs will become applicable from 12 January 2025 for medicinal products with new active substances for oncological indications and advanced therapy medicinal products (ATMPs). From 13 January 2028 onwards, manufacturers of orphan drugs will have to submit a dossier for the joint clinical assessments. All other medicines approved under the EU centralized procedure will be subject to JCAs from 13 January 2030.
Implication for the pharmaceutical industry: 2025 will fast be upon us, and so the pharmaceutical industry must start to consider the impact of the joint HTA. This will include thinking about the choice of comparator, the correct endpoints and appropriate patient subgroups for a joint EU clinical assessment. To aid with this, companies may wish to take the opportunity to have joint scientific consultation to help them better plan their clinical trials. All of this said, there is still a large amount of uncertainty surrounding how comparators, endpoints and subgroups will be chosen to satisfy the needs of all 27 member states and how the joint assessment will be incorporated into country level pricing and reimbursement decisions.
Prediction Two – Set up of the Coordination Group
Our prediction: The Coordination Group and the first subgroup on methodology will likely be set up by the end of 2022 and the stakeholder network will hopefully be operational from 2023.
What happened: In June, the Regulation on health technology assessment established the Coordination Group on Health Technology Assessment composed of Member States’ representatives, mainly from HTA authorities and bodies. This represented an important first step for the establishment of the joint clinical assessment and joint scientific consultation. This group is in two configurations, one for medicinal products and one for medical devices. The primary task of this group is to coordinate and adopt the joint HTA work and to adopt methodological and procedural guidance documents for joint work.
Implication for the pharmaceutical industry: The Coordination Group will be responsible for developing the framework that supports the joint scientific consultation and the joint clinical assessment. Therefore, it will be crucial for the pharmaceutical industry to follow their work closely to understand in detail how these processes will work. Further to this, the Coordination Group should act as a catalyst for national implementation, the success of this will better help the pharmaceutical industry understand the level of duplication of work which they may have to perform on the national level.
Prediction Three – Issuing of draft guidance
Our prediction: The coordination Group will start drafting guidance documents in 2022 and 2023.
What happened: In 2022, we have seen the release of a number of deliverables published which supports the EU HTA process (find them here). We have seen the publication of the template and certain guidelines for the joint clinical assessment. Further to this we have seen guidance on how manufacturers should interact with the joint EU HTA body and how the EU HTA body should interact with patients, health care professionals and other experts. Clarity has also been given on the scoping process and use of direct and indirect treatment comparisons following issuing of the guidance earlier this year.
Implication for the pharmaceutical industry: The industry is beginning to have a greater understanding of how the joint EU HTA process will work after the publication of several guides and templates. These should be studied to allow for the correct market access activities to take place for products expecting to go through the joint clinical assessment.
What else happened in 2022?
Initiation of joint scientific consultation: There was an open call for applications from the pharmaceutical industry for joint scientific consultations. This call closed at the end of August and the process for joint scientific advice began in October. Five joint scientific consultations will take place on medicinal products in parallel to EMA scientific advice. Amongst the selected there are three oncological and two non-oncological products: 5x First in class, 2x Orphan designation, 2x ATMP, 1x PRIME (priority medicines) and 1x SME (small and medium-sized enterprises).
Discontent from several stake holders: This year multiple stake holders have expressed their concern with the Joint EU HTA process. One notable one was a joint statement from European Confederation of Pharmaceutical Entrepreneurs (EUCOPE) and European Federation of Pharmaceutical Industries and Associations (EFPIA) – read our summary of it here. The largest concerns are that considerations have not been made on comments from key stakeholders, that the process may end up being a mismatch of current systems rather than an aligned process that successfully merges the HTA systems across the EU, and that the pharmaceutical industry themselves have not been asked for their involvement for the development of the process.
Expectations for 2023
In 2022, following the Regulation coming into force the joint EU HTA process has come on a lot and is really starting to take shape. Now, at the end of 2022, we are just two years away from the first products entering the process. So, what can we expect to happen in 2023?
- Publication dates for documents on the joint scientific consultation are expected in 2023. This is a difficult subject matter with many still concerned about how the correct comparators, endpoints, patient subgroups, and PICO can be advised upon, given that it must satisfy many country’s needs.
- Member states to familiarise themselves with the Regulation. Each state needs to understand the Regulation so that they can generate a framework for it to be supported in their country.
- Creating new legislation. Each member state needs to begin the process of ensuring that they have the correct legislation in place for 2025 so they can reap the benefits of the joint EU HTA process. With this, we may also begin to see the additional layers of assessment that some countries may wish to implement beyond the joint clinical assessment to aid them in making their pricing and reimbursement decisions.
- Fine tuning of the EU HTA process. Some of the concerns that have been raised so far need to be addressed. Namely, specifics must be given about how the HTA process can successfully integrate to give alignment on evidence requirements.
- Set up of the stakeholder group.
Conclusion
This year we have seen that the joint EU HTA process is being moulded into something tangible rather than the distant dream for which it remained for many years. Following the Regulation coming into force in January of this year it has been all systems go, with the Coordination Group being appointed followed by them leading in the publication of a host of guidelines and templates for the processes. However, there are still concerns from stakeholders as to the proficiency of the current processes and whether they will work in practice. Going forward, these wrinkles will have to be ironed out by aligning the individual country processes rather than haphazardly overlaying them. As this occurs, we can expect to see individual member states building a framework and writing legislation to support the Regulation for when it becomes applicable in 2025.
Sources:
- Regulation on Health Technology Assessment. European Commission. https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en. Accessed on 11th November 2022
- Member State Coordination Group on HTA (HTACG). European Commission. https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment/member-state-coordination-group-hta-htacg_en. Accessed on 11th November 2022
- Joint HTA Work. EUnetHTA. https://www.eunethta.eu/jointhtawork/. Accessed on 11th November 2022
- Joint Scientific Consultation (JSC). EUnetHTA. https://www.eunethta.eu/jsc/. Accessed on 11th November 2022
