Introduction
The 2021 EU HTA Regulation and the recent March 2024 Draft Implementing Act (the first of 6 implementing acts, see Figure 3) signifies a notable shift in how companies navigate the Health Technology Assessment (HTA) landscape.1,2 This regulation is designed to promote the harmonisation of clinical assessments at the EU level, with primary goals of 1) reduce redundant HTA activities, 2) promote efficiency in decision-making processes, and 3) reduce the time to access for patients.
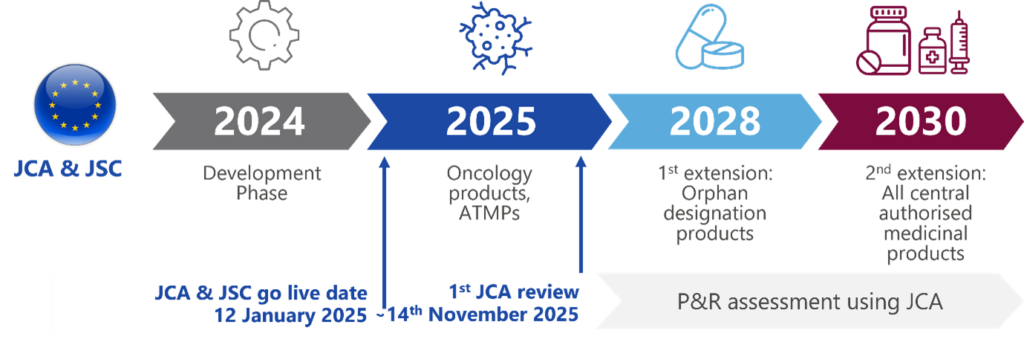
As the regulation introduces new elements like Joint Clinical Assessments (JCAs) and other significant modifications, companies are advised to proactively prepare. These changes will take effect in January 2025 in oncology and advanced therapy medicinal products (ATMPs), 2028 in orphan drugs, and all drugs by 2030 (see Figure 1). The EU HTA aims to advance patient-centred care, promote innovation, and address unmet medical needs on a global scale.1,2
Key changes in HTA landscape
Before we delve into the company preparation required, here are the key changes introduced by the EU HTA to be aware of:2,3
- Joint Clinical Assessments (JCAs):
JCAs are a central feature the EU HTA, designed to harmonise the evaluation of new medicinal products across the European Union. The Draft Implementing Act details procedures for conducting JCAs, including the preparation of assessment scope proposals, consolidation meetings, and finalisation of assessment scopes.
The JCA will not make value judgements or rank health outcomes, however the JCA dossier will require additional information compared to typical member state submissions. For example, epidemiological data will need to be provided for relevant EEA states, including “any profound differences between these states”, as well as substantial variations in clinical pathways between EEA states (including European clinical guidelines).
Patient and clinician involvement is a strong feature of the JCA, with the draft act specifying the selection process.
- Joint Scientific Consultations (JSCs):
Complementing JCAs, JSCs provide a platform for manufacturers to engage in early scientific dialogue with HTA bodies. JSCs enable manufacturers to seek guidance on study design, endpoints, and other critical aspects of clinical development, increasing alignment with HTA requirements.
JSCs will be considered by the JCA, meaning companies will need to report in their JCA dossier why they have deviated from the JCA advice provided. Considering this, whether to opt for JSCs and the weight given to EU HTA requirements need to be considered early stage of product development.
- Revised Submission Timelines:
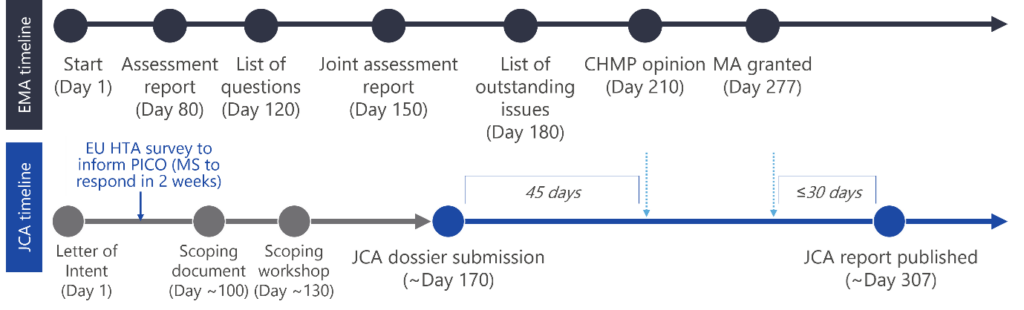
EU HTA happens in parallel to EMA submission (see Figure 2). The JCA timelines are strict, once the process has started the company will only be able to delay in limited cases (see article 12 of the Draft Implementing Act for more detail).2 Procedures for managing updates to therapeutic indications are detailed in article 16.2
What does the EU HTA actually mean for manufacturers?
Here’s a closer look at why these changes matter and the key strategic considerations companies should prioritise:5
Early development and timelines adjustments
- EU HTA happens in parallel to EMA submission.2 As mentioned, the JCA timelines are strict. This means it is imperative for companies to begin consideration of value proposition and EU HTA strategy development earlier on. For smaller companies, this may cause additional challenges due to resource limitations. Moreover, at this stage the clinical data may not be mature enough to support payer value.
- Shifting pre-launch activities and aligning internal processes with new timelines is essential. Companies should be prepared for earlier start dates and potentially longer durations due to increased complexity.
Resource management
- Increased workload demands strategic resource allocation and capacity planning. Companies need to reassess teams, possibly create new roles, and invest in developing HTA technical skills at the global and regional levels.
- Involving local teams is crucial to streamline HTA submissions and market access in individual countries. The need for intensified collaboration among global and regional teams, including regulatory, market access, clinical development, and health economics is paramount.
Perspective
Overall, as we’ve discussed above, the EU HTA will be introducing several changes and requires manufacturer preparation. Given this, it is unsurprising that the introduction of the EU HTA is being met with apprehension. Indeed, there are several potential challenges. One of these, which has been widely debated in recent months concerns the number of PICOs (population, intervention, comparators, outcomes). JCA subgroups, responsible for delivering JCA reports, should aim to limit PICOs to 2-3, however research by Remap Consulting has demonstrated there could be several more.6,7 Similarly,EFPIA findings have recently demonstrated potentially 57 PICOs in oncology.8
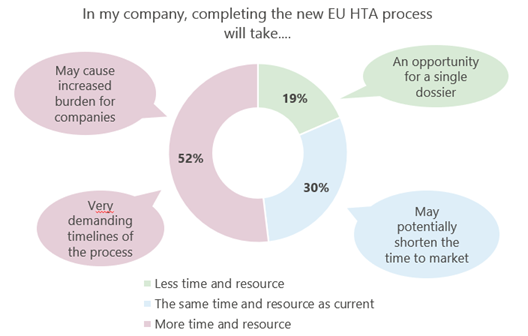
Despite challenges however, it is important to note that the EU HTA does also present some opportunities. According to a survey conducted by Remap Consulting, key highlights include the potential for a single dossier and potentially shorter time to market (see Figure 3). In addition, it is important to recognise that companies will still be able to employ similar methodologies as before, such as indirect comparisons, enabling them to maintain continuity with their previous approaches.
Many of the challenges that manufacturers will face with the EU HTA are already being tackled in current HTA assessments. These challenges encompass issues like immature overall survival data, trial comparators becoming irrelevant by trial end due to shifts in the treatment landscape, and the necessity for network meta-analysis.
Moreover, parallels can be drawn between this transition to a centralised EU HTA and the centralisation of individual regulatory assessments to the EMA in 1995. Despite initial scepticism, the EMA successfully reduced the costs drug companies incurred by having to win separate approvals from each member state, and provided harmonisation in drug availability across member states.
Conclusion
In conclusion, although these changes are not procedural shifts, they bring possibilities of reduced time and resource in the future. The changes do however necessitate a strategic recalibration of how companies approach market access, collaboration, resource management, and overall organisational readiness. The EU HTA impacts the entire development pathway, and staying agile amid uncertainties will be key to success.
Remap can support you and your company prepare for change and help you thrive under the EU HTA. For more information please reach out.
P.S. If you haven’t yet checked out our latest webinar “EU HTA: Moving from strategy to action”, watch on demand here!
Sources:
1. European Commission,. Implementing the Eu health technology assessment regulation. Europa.eu. Published 2023. Accessed March 18, 2024. https://health.ec.europa.eu/system/files/2023-09/hta_regulation-implementation_factsheet_en.pdf
2. European Commission – Have your say. European Commission – Have your say. Accessed March 20, 2024. https://ec.europa.eu/info/law/better-regulation/
3. From Theory to Practice: Implementing the EU health technology assessment regulation. Public Health. Accessed March 18, 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-01-30_en
4. Craddy P, Foxon G. Webinar: Implications of the EU HTA process for manufacturers. Remap Consulting. Published April 27, 2023. Accessed March 18, 2024. https://remapconsulting.com/hta/implications-of-the-eu-hta-process-for-manufacturers/
5. Bean S. What are Joint Scientific Consultations and why they are important for manufacturers to consider? Remap Consulting. Published April 13, 2023. Accessed March 18, 2024. https://remapconsulting.com/hta/what-are-joint-scientific-consultations-and-why-they-are-important-for-manufacturers-to-consider/
6. Boland L. EU HTA and what it means for manufacturers. Remap Consulting. Published April 20, 2023. Accessed March 15, 2024. https://remapconsulting.com/hta/eu-hta-and-what-it-means-for-manufacturers/
7. Boland L. ISPOR Europe 2023: Can just three PICOs be feasible for oncology assessments with the joint EU HTA framework, whilst considering all 27 member states specificities? Remap Consulting. Published October 25, 2023. Accessed March 15, 2024. https://remapconsulting.com/hta/ispor-europe-2023-can-just-three-picos-be-feasible-for-oncology-assessments/
8. EU HTA Regula-on for oncology medicines: Learnings from a simula-on on the impact of proposed EUnetHTA21 methods. Efpia.eu. Published March 2024. Accessed April 10, 2024. https://www.efpia.eu/media/qrjah2ij/efpia-evidera-research-on-eunethta21-methods.pdf
