The Joint Nordic HTA Bodies (JNHB), introduced in 2017 as known as FINOISE, was launched by Nordic authorities responsible for HTA assessments with the purpose of increasing and developing HTA collaboration and streamlining access to new medicines. Consisting of the Finnish Medicines Agency (Fimea), the Swedish Dental and Pharmaceutical Benefits Agency (TLV), the Norwegian Medicines Products Agency (NOMA), the Danish Medicines Council (DMC) and the National University Hospital of Iceland (Landspitali), the collaboration includes the assessment of both relative effectiveness and economic evaluation.
Recently, the JNHB have produced draft guidelines to provide information on the process by which Nordic joint HTA will function. Key excerpts from the guidelines are summarised below:
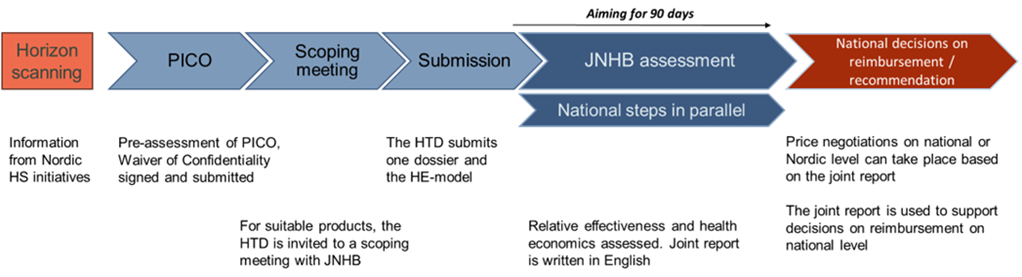
- PICO proposals submitted, with a Nordic perspective, will have a pre-assessment conducted by the JNHB to ensure current treatment practices are sufficiently comparable
- JNHB members will select the assessor, co-assessor, and reviewer together, and any HTA bodies for which the product is outside their remit will function as an observer in the process
- JNHB submissions should follow the Swedish national procedure and be submitted to the TLV
- The first draft of the assessment should identify and address key questions in the evaluation and report key uncertainties and their resulting impact on the results
- The second draft should be submitted close to finalisation, including the JNHB base case, with scenario and sensitivity analyses, and drafts of country-specific analyses if conducted
- After formal approval from JNHB agencies, national bodies may sign off the finalised report for their respective countries, who publish the report in line with national procedures
The collaboration hopes to support decision-making processes in the member countries to enhance the quality and efficiency of HTA reports, facilitate resource and knowledge sharing among Nordic HTA bodies, and reduce administrative burden for the pharmaceutical industry.
Sources:
- The Joint Nordic process. JNHB. https://jnhtabodies.org/working-with-jnhb. Accessed 10th July 2024
- Nordic collaboration through JNHB. Fimea. https://fimea.fi/en/development/therapeutic_and_economic_value_of_medicines/nordic-collaboration-finose-. Accessed 10th July 2024
- Joint Nordic HTA-Bodies Process Guideline. JNHB. https://jnhtabodies.org/media/yn5fkfza/jnhb-process-guideline.pdf. Accessed 10th July 2024
- FINOSE Rebrands as Joint Nordic HTA-Bodies. Navlin Daily. https://www.navlindaily.com/article/21887/finose-rebrands-as-joint-nordic-hta-bodies. Accessed 10th July 2024
