Regularly newspaper headlines talk about struggling healthcare systems, restricted healthcare budgets and increasing treatment costs. They also highlight, particularly in Europe, that innovative treatments do not reach patients as payers deny market access. Key drivers for market access rejections are high perceived product price, large estimated budget impact and/or concerns around clinical efficacy and safety as well as patient population. Managed entry agreements (MEAs) are one potential way of reducing payer concerns and uncertainties and enabling patient access.
What are MEAs?
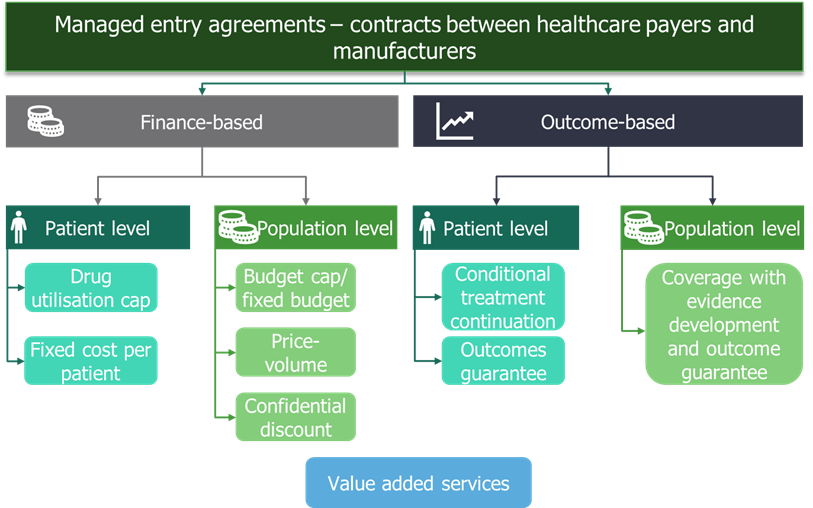
MEAs are also known by other names such as risk-sharing agreements, value-added services, result-base payments and patient access schemes. They are contracts between manufacturers and payers that help mitigate payer uncertainty and allow faster access to new innovative healthcare technologies. Finance- and price-based MEAs have been around for many years in various forms. However, more recently outcome-based MEAs have been introduced (Figure 1).
Types of MEAs
The manufacturer can enter:
- A finance-based MEA that addresses the payer’s budget impact concerns by reducing the net price of a product
- An outcome-based MEA that addresses the payer’s concern around the clinical value such as efficacy and safety.
Both categories can operate on an individual patient level or at the population level resulting in four subcategories (Figure 1). Within these subcategories, there are different approaches a manufacturer can adopt when introducing a MEA (Figure 1).
Advantages and disadvantages of MEAs
All MEAs pose advantages and disadvantages to both the manufacturer and the payer. MEAs require time and resources to develop, initiate and implement. Some MEAs (particularly outcome-based MEAS) require additional monitoring and/or data collection across the product lifecycle.
Across the globe, payers and manufacturers are more familiar with finance-based MEAs. These types of agreements are typically less complex and relatively easy to implement, particularly at the population level. Finance-based MEAs generally address the payer’s concerns around budget impact. The agreements do not affect the list price of the product and therefore do not impact prices in other countries. Manufacturers need to be careful when negotiating finance-based MEAs to mitigate the potential financial downside of the agreements.
Over the past two decades, payers and manufacturers have experimented with outcome-based MEAs with varying degree of success. Outcome-based MEAs typically link the payment the manufacturer receives for the drug to treatment success. As such, outcome-based MEAs are generally more complex to implement and execute than finance-based MEAs as they require the treatment success to be monitored. In addition, outcome-based MEAs might also require additional data collection. For payers, outcome-based MEAs reduce the risk of funding a product that provides less clinical benefit than anticipated. While for manufacturers, the financial return can increase if the product performs better than expected.
When should manufactures opt for MEAs?
Even though MEAs have become more widespread and there are many examples, MEAs should be the exception rather than the rule. Manufacturers should do their utmost to ensure that their clinical development program is robust, providing the necessary evidence for payers to evaluate the product and its value. In addition, the price should reflect the product’s perceived value.
Finance-based MEAs can be used for products with a predicted high budget impact such as:
- Products with high cost and low volume
- Products with low cost and high volume.
Outcome-based MEAs are appropriate under specific conditions:
- The new treatment is innovative and addresses a high unmet need
- The clinical benefit is associated with significant uncertainty
- The clinical benefit shown in clinical trials might not reflect the benefit in real-world clinical practice.
When developing a MEA the manufacturer needs to ensure that:
- The type of MEA is appropriate for the competitive environment and company aspirations for the product
- There is a clear rationale for the MEA
- The MEA addresses payer concerns and uncertainties
- The national/regional/local healthcare infrastructure can support implementation of a MEA.
To ensure the success of a MEA manufacturers should engage early with payers, health technology assessment (HTA) bodies and other stakeholders. They must also secure alignment within their organisations (e.g. market access, clinical, finance, health economics, legal) as a wide skillset is required to successfully implement agreements. Early preparation is a must.
In summary, adoption of MEAs is becoming more widespread, both across markets and the types of MEAs available. Manufacturers and payers see MEAs as useful tools to allow access to innovative pharmaceuticals where there is too much uncertainty over the value of the product, either from a financial or clinical perspective.