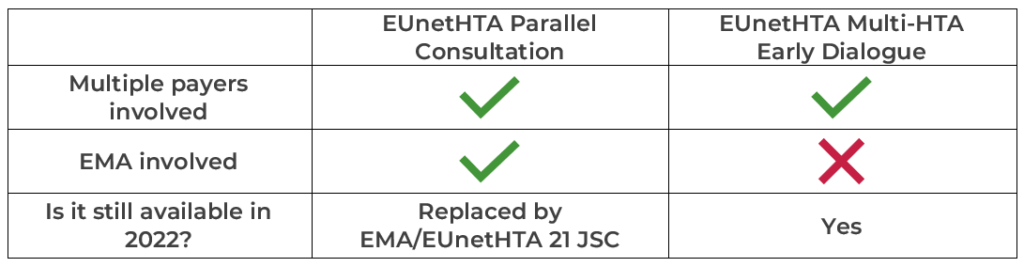
Introduction
Achieving access to patients for innovative treatments is becoming a significant challenge for manufacturers due to the increasingly complex regulatory and reimbursement environment. Historically, manufacturers have been proactive in seeking advice from regulators but have struggled to determine the payer evidence requirements. Today they have the opportunity to receive early scientific advice from individual local payers (e.g. NICE in the UK, G-BA in Germany, HAS in France) or undergo a parallel scientific advice process with multiple payers and/or regulators. Until recently the European Network for Health Technology Assessment (EUnetHTA) has offered two options for early dialogue/advice for pharmaceuticals: European Medicines Agency (EMA)-EUnetHTA Parallel Consultation and EUnetHTA Multi-HTA Early Dialogue1. Moving forward, the EUnetHTA Parallel Consultation will be replaced by the Parallel EMA/EUnetHTA 21 Joint Scientific Consultation (JSC).
The centralised joint EU health technology assessment (HTA) will be implemented in a staged approach over the next decade and would make it mandatory for manufacturers to submit a European level HTA dossier for joint clinical assessment1. Moving forward, manufacturers will need to have more than ever a single, reliable, parallel, and streamlined early advice (dialogue) process which provides them with relevant insights that would feed into manufacturers pivotal trial design in order to meet EMA and all individual EU member state requirements in these joint HTA assessments.
What is the Early Dialogue within EUnetHTA?
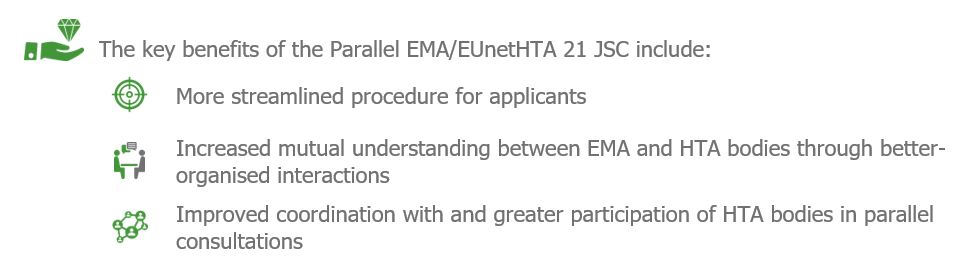
The EMA began to offer a parallel Joint Scientific Consultation (JSC) with the EUnetHTA 21 consortium. The aim of these consultations is to help pharmaceutical and biotech companies to receive feedback from regulatory and HTA authorities from EU Member States on their development strategy for marketing authorisation and reimbursement at the same time. By doing so manufacturers can ensure their evidence generation strategy is aligned with the requirements of regulators and HTA bodies. This facilitates and expedites patient access to innovative treatments, some of which save patients from life-threatening conditions2.
This Parallel EMA/EUnetHTA 21 JSC has replaced the former Parallel Scientific Advice procedure by EMA and HTA institutions, where drug developers had to contact individual HTA bodies in each Member State separately. The scope and fees for this procedure will remain the same as for standard scientific advice offered by the EMA3.
What is the main purpose of the Parallel EMA/EUnetHTA 21 JSC?
The parallel JSCs between EUnetHTA and EMA provide non-binding scientific advice before the initiation of pivotal clinical trials for medicines, which typically happens after the completion of their proof-of-concept study. Medicine developers will also benefit from patient and clinical expert contributions, who will be involved in the parallel consultation process and their opinion will be reflected in the final recommendations provided by HTA bodies. One of the main goals of the parallel EMA/EUnetHTA 21 JSCs is to enable the exchange of information between the manufacturer, EMA and HTA agencies as early in the drug development process as possible. This would give manufacturers enough time to consider the feedback on their clinical development programs and align them with the requirements of the HTA agencies across EU member states. Thus, manufacturers can enhance their evidence generation strategy by optimising trial design – e.g. choice of comparators, relevant outcomes, quality of life, patient (sub)groups etc. This applies not only for pivotal trials but also any post launch studies that pharmaceutical companies plan to undertake. If the HTA bodies across the EU member states cannot reach a consensus, the manufacturer will be notified so they have time to tailor their strategy to individual country requirements to obtain access for their products. In the period 2021-2023 there will be between six and eight JSCs for medicinal products4.
Who will lead and which are the key stakeholders in the EUnetHTA 21 JSCs?
The Committee for Scientific Consistency and Quality (CSCQ) is in charge of the JSC process and oversees all activities associated with it. Also, CSCQ is responsible for the consistency and quality of the deliverables in line with the EUnetHTA 21 service contract. Key stakeholders and contributors to the scientific advice are AEMPS (Spain), AIFA (Italy), G-BA (Germany), HAS (France), INFARMED (Portugal), KCE (Belgium), NCPE (Ireland), NIPN (Hungary), NOMA (Norway), TLV (Sweden) and ZIN (The Netherlands).
How will products be selected for EUnetHTA 21 JSCs?
The number of products which will benefit from JSCs at EUnetHTA is currently limited. However, it is expected that manufacturer interest in participating in EUnetHTA 21 JSCs will rise in the future. The key prerequisite for a JSC is that the clinical studies, for which advice will be sought, are still in the planning phase. Additionally, candidates will be selected based on several criteria defined by the EU HTA regulation, including5:

Oncology treatments and advanced therapy medicinal products (ATMPs) for which there are no recent HTA evaluations on similar products will be prioritised in the selection of applications.
When will Parallel EMA/EUnetHTA 21 JSCs be initiated?
EUnetHTA 21 launched the first Open Call for JSCs in November 2021 and is launching the second Open Call in June 2022, which will be open from 6th June 2022 to 31 August 2022. The final decision on product selection and notification of manufacturers will take place in September 2022. It is anticipated that initially five products will undergo these parallel consultations, which will be initiated in November 2022.
| Event | Date |
| Open Call published online | 6th June 2022 |
| Open Call open | 6th June – 31st August 2022 |
| Selection of products | 12th September 2022 |
| Information sent to applicants | 14th September 2022 |
| Start of the JSCs | 1st November 2022 |
What would this mean for the UK?
Many experts in the field ask themselves what the impact of the UK being out of the EU will have on Joint Scientific Advice process/EUNetHTA. The UK has always played a vital role in the parallel consultations between regulators and multiple HTA agencies across Europe. Since the creation of EUnetHTA, the National Institute for Health and Care Excellence (NICE) was one of the key stakeholders whose input was often sought in these consultations due to its extensive experience in conducting HTA assessments. Brexit, however, has a notable impact on the integration of NICE and UK, as a whole, within the European regulatory and reimbursement activities associated with medicines. As a result of Brexit, the following consequences apply to the UK:

Although pre-Brexit NICE was heavily invested in parallel consultations with EMA, moving forward it will no longer take part in parallel consultations between HTA agencies and/or the EMA6.
NICE have also announced changes in their approach to delivering an early scientific advice. From now, they will aim to execute this consultation in similar timelines to the EMA process. Thus, companies could undergo an early scientific advice with NICE during the same time when they undergo a regulatory advice from the EMA. NICE’s offering will also remain a great opportunity for these manufacturers, who have applied for a parallel consultation with the EUnetHTA and have been rejected6.
To minimise the impact of Brexit on patient access to medicines in the UK, NICE will have to continue to work in close collaboration with the MHRA to facilitate pharmaceutical product development and access as much as it can. Considering the UK is now outside the EU, it may be recognised as an early launch country due to its relatively high drug prices, which facilitate subsequent launches in terms of international reference pricing (IRP). If NICE and the MHRA offer a robust parallel fast-track approval process, the UK may come across as a great opportunity for manufacturers to ensure early approval in the country. This would then allow manufacturers to generate real world evidence (RWE) which could be valuable for their European and Global HTA submissions.
Conclusion
In conclusion, the gradual integration of joint clinical assessments as part of the European joined HTAs will likely increase the demand of early parallel consultations with regulators and HTA bodies because manufacturer will no longer need to contact the HTA agency of each EU member state individually. The new JSCs seem to be a pragmatic and streamlined way of obtaining valuable insights into drugs evidence generation and clinical development strategy. One of the anticipated challenges will be how to qualify for these parallel consultations. Initially only a few products, such as oncology treatments and ATMPs, will be selected for the JSCs, which means that it would be difficult for other products to qualify for an early dialogue. However, it is certain that with the HTA process being likely to become more and more centralised in Europe, manufacturers would need to allocate more resources and time to ensure they are aligned with the European HTA requirements.
Sources:
- 2022. Early Dialogues for Pharmaceutical Products – EUnetHTA. https://www.eunethta.eu/early-dialogues-for-pharmaceuticals/ [Accessed 23 May 2022].
- European Medicines Agency. 2022. Parallel joint scientific consultation with regulators and health technology assessment bodies – European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-advice-protocol-assistance/parallel-joint-scientific-consultation-regulators-health-technology-assessment-bodies [Accessed 23 May 2022].
- 2022. Parallel Consultation – EUnetHTA. Available at: https://www.eunethta.eu/jointhtawork/parallel-consultation/ [Accessed 23 May 2022].
- 2022. Joint Scientific Consultations (JSC) – EUnetHTA. Available at: https://www.eunethta.eu/jsc/ [Accessed 23 May 2022].
- 2022. Joint Scientific Consultations (JSC) – EUnetHTA. Available at: https://www.eunethta.eu/jsc/#:~:text=Parallel%20EMA%2FEUnetHTA%2021%20Joint%20Scientific%20Consultations%20(JSCs)%20provide,of%20future%20HTA%20assessment%20%2F%20re%2D [Accessed 23 May 2022].
- 2022. How will NICE fare in a post-Brexit world? – pharmaphorum. [online] Available at: https://deep-dive.pharmaphorum.com/magazine/market-access-2021/nice-post-brexit-icon/ [Accessed 23 May 2022].
