When faced with evaluating alternative treatment options, healthcare decision makers must consider multiple, often conflicting, criteria. Multi-criteria decision analysis (MCDA) is a potentially useful tool that can be used to support such decision making. For the past few years, the options and feasibility of using MCDA for health technology assessments (HTA) have been explored. Both the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and National Institute for Health and Care Excellence (NICE) have published reports that describe and evaluate MCDA’s applicability for HTA.
What is MCDA?
In the literature, MCDA is defined as “an umbrella term to describe a collection of formal approaches, which seek to take explicit account of multiple criteria in helping individuals or groups explore decisions that matter”. At its core, MCDA is useful for:
- Dividing the decision problem into smaller, more understandable parts
- Analysing each part
- Integrating the parts to produce a meaningful solution.
During the analysis step, a transparent, mathematical approach is often adopted. This necessitates that evidence is expressed in a quantifiable, numerical way.
How do MCDA processes compare to current HTA processes?
In the UK, NICE has compared the MCDA approach to its current appraisal process (Figure 1) to determine whether it is feasible to include MCDA in its appraisal process. The main difference was seen in the decision-making stage. As stated above, for MCDA evidence should be quantifiable as it will be used in mathematical models. In contrast, during the current NICE process the evidence is evaluated in a deliberative manner using health economic analysis and other criteria.
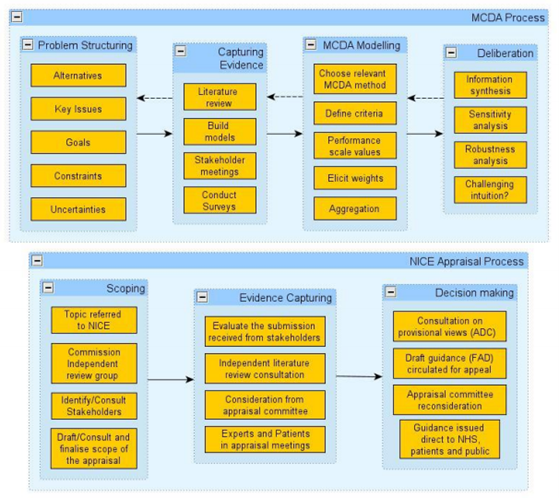
Current use of MCDA in healthcare decision making and HTA
MCDAs have already been used in Europe and worldwide to inform healthcare decision making. For example, the Lombardy region in Italy has included MCDA in its HTA process to decide on the introduction and delisting of health technologies. Within the UK, MCDA have also been used by a Health England Leading Prioritisation (H.E.L.P) study to prioritise investment in preventative health interventions. In addition, NICE’s highly specialised technology (HST) appraisal process and Scottish Medicines Consortium’s (SMC) Patient and Clinician Engagement (PACE) program for ultra-orphan drugs have features of MCDA processes, though both do not rely on mathematical models.
The challenge of implementing MCDA in HTA
Implementation of MCDA in HTA submission requires identification of criteria that are (a) relevant to decision makers and (b) quantifiable. These criteria should be clearly defined, judgmentally independent and scalable. In addition, they should be relevant to the decision problem, non-redundant, understandable and feasible. The Evidence and Value: Impact on Decision Making (EVIDEM) framework has developed a list of fifteen explicit quantitative criteria that are grouped into four clusters (quality of evidence, disease impact, intervention and economics) and six implicit qualitative criteria. However, it is the ranking of these criteria that still possess a challenge.
Whilst some attributes (e.g. budget impact and economic) readily lend themselves to the numerical approach of MCDA, other attributes are much hard to quantify (e.g. additional benefit over comparator, patient quality of life or care giver burden) and could result in being subjective, potentially undermining the whole approach for MCDA. The transparency associated with MCDA may also pose challenges for payers who may be unable to fund new treatments, whereas previously the not recommended decisions were made on the basis of less tangible parameters.
Manufacturers could consider implementing MCDA in their pricing and market access strategies
In some aspects, it seems that MCDA is an almost natural extension of current HTA processes. As such, it can be anticipated that in future more healthcare decision makers may include MCDA or MCDA-like processes in their HTAs and decision making. Manufacturers may want to consider incorporating relevant and measurable criteria, such as identified by the EVIDEM framework, into their pricing and market access strategies to prepare for the future use of MCDA within HTA and patient access decision making. Even if MCDA is not formally included into HTA the identified criteria are still be important to decision makers and inform their conclusions.
