What are the implications for patient access for new pharmaceutical products?
Given the unprecedented global shutdown occurring in response to COVID-19, payers and HTA bodies are struggling to adjust to the new way of working. What impact is this having on existing drugs that are currently undergoing pricing, reimbursement and health technology assessments? How has each country adapted to the challenges?
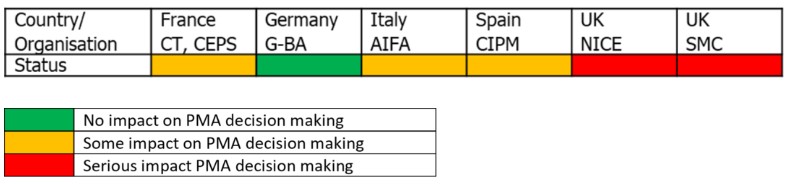
Summary of how HTA bodies decision making for new medicines are affected by COVID-19
UK: The National Institute for Health and Care Excellence (NICE)
NICE have announced they are stopping all non-critical NICE assessments. Only “therapeutically critical” assessments and COVID-19 diagnostic or therapeutic interventions will be undertaken in the short term, due to the pressures put on the NHS. In practice, this means all cancer medicines and a selected few non-cancer medicines. Technology Appraisal committee meetings are continuing to be held (except for April). However, these will be conducted virtually.
If your product is not therapeutically critical, NICE will be in contact with you to discuss the impact and revised timetables.
UK: Scottish Medicines Consortium (SMC)
The SMC have cancelled the New Drugs Committees (NDC) and SMC meetings due to take place in March, April and May 2020. This is to enable committee members and staff to support work aligned with COVID-19. New medicine submissions that are currently in progress will be supported by a core team, as well as COVID-19 treatment.
No date is scheduled for the resumption of meetings at the moment.
France: Commission de la transparence(CT) and Comité Economique des Produits de Santé (CEPS)
Haute Autorité de Santé (HAS) has announced that the Commission de la transparence meetings are proceeding according to schedule whilst the Comité Economique des Produits de Santé is still in discussions with pharmaceutical companies they are currently not meeting to vote on pricing recommendations. This is due to the fact that they currently do not have a process in place to enable remote voting. As such, the weekly CEPS committee meetings have been postponed for the foreseeable future. The CT met on March 18th by video conference and can assess any new COVID-19 treatment. During this time, HAS has stated that it will continue to focus on essential activities, but has not detailed what that means in practice. In reality, there is still a large backlog in applications, with the CT currently taking the CT about seven months to make a clinical assessment. As such, the current crisis is likely to add to this delay.
Germany: Gemeinsamer Bundesausschuss (G-BA)
The G-BA stated that its meeting will be able to occur virtually, using videoconferences and written decision-making. As such, it does not expect any delays in G-BA assessments or pricing negotiations. A raft of new legislation has been introduced to support the COVID 19 pandemic.
Italy: Agenzia Italiana del Farmaco (AIFA)
AIFA are still publishing agendas for the Comitato Tecnico Scientifica (CTS – clinical assessment body) and Comitato Prezzi e Rimborso (CPR – pricing body) and the meetings are occurring remotely e.g. CPR meeting on 24-26 March). AIFA has also established a Coronavirus crisis unit within the CTS to support, asses and provide guidance on treatments and approaches for COVID-19.
Given the impact of COVID-19 on Italy, delays to the pricing and reimbursement process are likely due to staff shortages. Price negotiations may be more protracted due to them having to take place virtually. Similarly, significant delays in regional market access are expected due to staff shortages and prioritization of resources elsewhere to manage the pandemic.
Spain: Dirección General de Farmacia y Productos Sanitarios (DGFPS) and La Comisión Interministerial de Precios de los Medicamentos (CIPM)
The Therapeutic Positioning Report Coordination Group (GCPT committee) are still holding meetings (virtually) on a monthly basis and, for the time being, therapeutic positioning reports (TPRs) are still being prepared. However, it is expected that these reports will take longer to prepare due to the collaborative nature of report development and need for clinical input. The Dirección General de Farmacia y Productos Sanitarios and La Comisión Interministerial de Precios de los Medicamentos pricing and reimbursement committee are still meeting with pricing negotiations on-going and scheduled for 1st April. As with Italy, Spain has a devolved healthcare system and as a result significant market access delays at a regional level are likely.
COVID-19 is having a significant impact on how governments and healthcare systems provide access to new medicines. Whilst most are trying to mitigate the impact by virtual working, the effect of the pandemic on staff and workload means it is likely that negotiations and decision making will take longer to achieve. Some HTA bodies like NICE have only prioritised therapeutically critical medicines, whilst others such as the SMC have suspended decision making altogether.
In the short term, this means that manufacturers are likely to experience longer time to market for new pharmaceuticals currently in the review process or new treatments that are just about to be submitted. In the longer term, once countries recover from the pandemic, it is likely the new pharmaceuticals will come under increasing price pressure, as governments seek to recover the extraordinary funding that has been used to support their economies.
