Obtaining payer scientific advice to optimise trial design and maximise product value
Supporting in identifying and obtaining payer scientific advice to inform a Phase III trial design.
The challenge
Our client had in-licensed a new product that they were seeking to launch in the EU, following a recent launch in the US. Differences in clinical practice between the two regions meant that an additional Phase III study in a narrower indication was required. Scientific advice was sought from HTA bodies across the EU5 to ensure that the trial design best captured the value of the product, and supported patient access at a value maximizing price.
The solution
Remap Consulting implemented a tailored four step approach to guide the client team through the Payer Scientific Advice (PSA) process from start to finish.
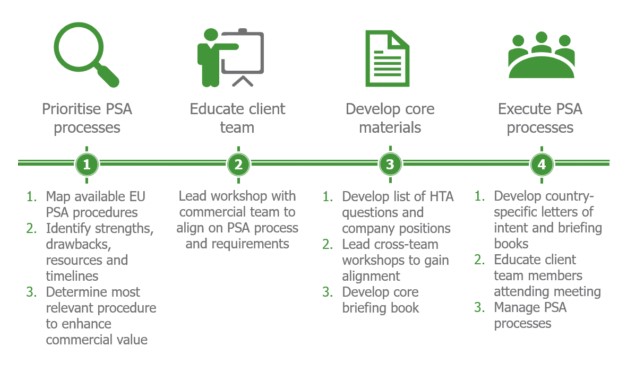
The benefits
A strong partnership was developed with the client, which greatly increased their understanding of the various PSA processes and requirements across the EU.
The core materials were developed by Remap Consulting to address the specific requirements of the HTA bodies, minimizing the time and effort needed from the client team. Wider client feedback was incorporated throughout this process, ensuring internal buy-in to the completed materials.
The client gained important insights from the HTA bodies during the PSA process, enabling strategic decisions to be made to help improve the Phase III study to meet the payer needs.
Testimonial
Thank you for the great job done on the briefing book. We sent it over to our colleagues who seem to be very satisfied with it.
Company Market Access Manager
