The Institute for Clinical and Economic Review (ICER) in the US has been making headlines, with its recently published “value based” Health Technology Assessments (HTAs) of newly launched drugs, such as Repatha and Entresto. These reports suggested that the current drug prices are too high and that the ex-factory price should be reduced by 17 ¬- 85% to justify the broad label population according to the label. How did ICER gain such prominence and what are the future implications for US HTAs and drug prices in general?
ICER was founded in 2007 as a not-for-profit organisation dedicated to improving the interpretation and application of evidence in the US health care system. It gained widespread attention with its assessment of the hepatitis C drugs in early 2015, where it encouraged payers to negotiate prices vigorously by leveraging the availability of multiple comparable treatments. Shortly afterwards, ICER launched a new program called the Emerging Therapy Assessment and Pricing (ETAP). ETAP received a $5.2 million grant to develop another 15 -¬ 20 reports over the next 2 years. The treatments to be assessed will be selected based on the potential to significantly change patient care and healthcare budgets. The PCSK9 inhibitors, Repatha (evolocumab) and Praluent (alirocumab), were the first drugs to be assessed.
The ETAP program aims to produce authoritative assessment and price benchmark reports at or near the time of FDA approval. The reports will include rigorous analyses of evidence on clinical effectiveness, cost-effectiveness, and potential budget impact. These analyses will also be combined, through the ICER value framework, to calculate in an objective, transparent fashion a “value-based price benchmark” for each therapy. Specifically, there will be assessments of two different types of value: “care value” and “provisional health system value.”
Care Value
Care Value consists of four different parameters:

- Comparative clinical effectiveness undertakes an assessment of the net health benefit and evidence base of the new treatment.
- Incremental costs per outcomes achieved calculates the Quality Adjusted Life Years (QALYs) gained from the new treatments, with the following thresholds:
- <$100,000/QALY = High care value
- $100,000 – 150, 0000/QALY = Intermediate care value
- >$100,000/QALY = Low care value
- Other benefits or disadvantages focuses on benefits to patients, caregivers or the health system that would not have been consider in the comparative clinical effectiveness assessment.
- Contextual considerations give importance to the disease severity and the current treatment options available.
Provisional Health System Value
The provisional health system value aims to assess the impact of the long-term care value of a new treatment with an analysis of its potential short-term budget impact.
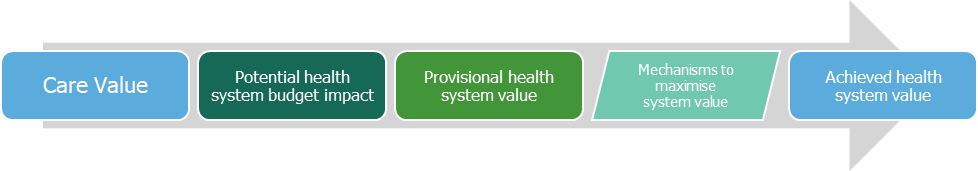
The budget impact is the estimated net change in total health care costs over an initial 5-year time-frame. ICER considers that a budget impact would be too high when the net cost increase per individual new treatment would contribute to growth in overall healthcare spending greater than the anticipated growth in national GDP plus 1%. The Care Value and Health System Value outcomes are discussed and voted on at a public meeting resulting in high, intermediate or low classification. This contributes to the value-based price benchmark, which is typically in the $100,000 – 150,000/QALY range. For a more detailed explanation of ICER methodologies, see http://www.icer-review.org/wp-content/uploads/2014/01/Slides-on-value-framework-for-national-webinar1.pdf
ICER’s assessments of PCSK9 inhibitors Repatha and Praluent proposed a price of $2,177 per year, as opposed to the current price ~$14,000 per year ex-factory list price. ICER stated that the treatments delivered “moderate certainty that PCSK9 treatment provides a substantial or incremental net health benefit”, but the price would have to be reduced by 85% to justify the broad label population that had been approved. It also recommended insurers use prior authorisations or retry patient on statins if price discounts for the PCSK9 inhibitors were not forth coming. Since then, reviews have been conducted for Entresto in heart failure and Nucala in asthma, with ICER suggesting a 17% and 76% price reduction respectively to achieve a value-based price. Both reviews received significant media coverage, but the actual impact of ICER’s review on health insurers is harder to gauge. Repatha, Praluent, and Entresto have all agreed outcomes-based pricing deals with major US insurer, but whether ICER’s analysis had any impact is unclear.
What is the future for ICER in the US?
Whilst HTA is a relatively new discipline in the US and ICER’s advice is non-binding, ICER does have some major advantages in being non-profit and gaining significant media coverage. The methodology used by ICER’s is less robust than that used by other HTA institutions (such as NICE), but the reports come at an opportune moment, given the highly politicised pharmaceutical pricing discussions that are ongoing. Whether insurers will utilise ICER’s reports is an ongoing debate, but given that the reports are widely available and broadly applicable, it would be surprising not to see them added to insurer’s armoury.
Also, whilst ICER’s assessments do consider QALYs (similar to NICE) it is not the defining metric, with the value-based price benchmark being preferred. This can help manufacturers move the pricing discussion forward to focus on areas of unmet need and subpopulations, where value is easier to demonstrate and price should be less of a topic. It will be interesting to see how this is reflected in future ICER reports, particularly the class reviews of relapsed multiple myeloma, non-small cell lung cancer, and multiple sclerosis that are scheduled for 2016.
Whether ICER will become an integral part of the US healthcare landscape in the future remains to be seen, but the signs are encouraging. It has demonstrated that there is a need for HTA in the US, that methodologically HTA can be undertaken and that health economics can be utilised. For manufacturers, this is not necessarily the end of free pricing, but another step along the road towards a potentially more regulated price environment.
