The 2019 Voluntary Scheme: a road to faster patient access and a resource challenge for NICE
As of 1st January 2019, the 2014 Pharmaceutical Price Regulation Scheme (PPRS) has been succeeded by the 2019 Voluntary Scheme for Branded Medicines Pricing and Access. Like its predecessor, the new Voluntary Scheme will be in place for five years and expire on 31 December 2023. The new scheme has been agreed by the Department of Health and Social Care, the ABPI, manufacturers who have joined the voluntary scheme, and for the first time, NHS England. Although not included in negotiations, the National Institute of Health and Care Excellence (NICE) will play a central role in supporting and operating the 2019 Voluntary Scheme, with a key goal of the new scheme being to guarantee “more and faster NICE appraisals for new medicines”. Companies which have chosen not to join the Voluntary Scheme are instead subject to the “The Branded Health Services (Costs) Regulations” (previously known as the “statutory scheme”) which was last amended separately to the Voluntary Scheme in early December 2018.
NICE value assessments: greater demand and faster timelines
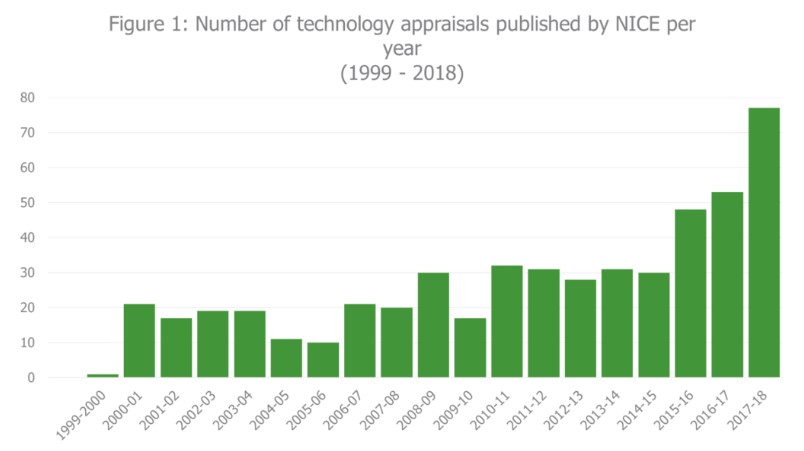
One of the most significant changes stated in the Voluntary Scheme, is the requirement for all new active substances in their first indication and significant indication expansions, to undergo a NICE appraisal (unless there is clear rationale to not do so). It is expected that this will be achieved by April 2020. Previously, NICE has only conducted appraisals for products with potential for significant health benefit, significant impact on NHS resources or where it was considered there was potential for NICE to add value by issuing guidance. In recent years, NICE have faced a significant increase in number of technology appraisals conducted per year, growing from 31 appraisals in 2013-14 to 75 appraisals in 2017-18 (Figure 1). The introduction of appraisals for all new active substances is likely to lead to further growth in this trend.
In addition to the greater demand for technology appraisals, NICE is also likely to face demand for earlier evaluations. As with the previous PPRS, sales of branded medicines are capped at an agreed level of growth, with any growth in sales above this level resulting in payments by the scheme members to the Department of Health. However, unlike the previous PPRS, the 2019 Voluntary Scheme makes new active substances and significant indication expansions exempt from company sales for the purpose of rebate payments for 36 months from the date of licence (backdated to January 2018). This change is likely to encourage manufacturers to aim for an earlier launch date in the UK and hence, earlier NICE submission. At the Westminster Health Forum event on Specialised Commissioning in December 2018, Meindert Boysen (Centre for Health Technology Evaluation Director at NICE) stated this change is expected to result in an increased demand for early appraisals and has the potential to cause resource challenges for NICE.
The 2019 Voluntary Scheme has also committed to providing faster patient access, with almost six months earlier access expected. As part of this commitment, NICE will aim to achieve equivalent appraisal timelines for non-oncology and oncology treatments. Since July 2016, oncology products have been subject to shorter appraisal processes than non-oncology products, with NICE aiming to publish final guidance for cancer drugs within 90-days of market authorisation. The Voluntary Schemes’ goal of achieving faster patient access and equivalent timelines across indications ties in nicely with the new shortened single technology appraisal process announced by NICE in April 2017. This new process aims to publish final guidance close to market authorisation and is currently being rolled out.
Enhanced horizon scanning to allow earlier referral for NICE appraisal and greater long-term planning for the organisation is also proposed to aid faster access. All scheme members will be required to commit to providing timely, accurate and comprehensive information on products in development. Similarly, the Voluntary Scheme has also emphasised a need for greater early engagement with manufacturers, in order to allow early clarification of uncertainties for all parties. NICE and NHS England will work together to lead development of both an approach to optimise horizon scanning and a “joined-up approach” to facilitate early engagement with manufacturers.
Implications
Overall, the 2019 Voluntary Scheme has taken significant measures to reduce the time taken for patients to gain access to new medicines. However, it is likely that NICE may face resource challenges to meet both the increasing demand for appraisals and the shortened timelines. It should be considered that the target for final guidance publication within 90-days of market authorisation, was only met by one oncology product between July 2016-Novemeber 2018 (Freedom of Information Request, November 2018). This was likely due to numerous factors including the late referral for guidance, late submission by pharmaceutical companies, appeals, delays in patient access scheme proposals, requests for further information post-submission or other factors. In addition, as yet, no products have completed the new shortened technology appraisal process announced in April 2018. Over the coming years, it will be interesting to see whether the new STA process and measures taken in the 2019 Voluntary Scheme is able to deliver faster patient access.
