The movement towards increasing patient involvement in HTA
Historically, patient involvement in health technology assessment (HTA) processes and decision-making has been limited. In recent years there has been a drive to reverse this for two main reasons:
- firstly, patient participation can enhance trust in the HTA decision-making process and acceptance of the resulting decisions;
- and secondly, patients are able to contribute valuable first-hand knowledge of living with a health condition[1]. Patients are the experts on quality of life and provide a different perspective on the impact of certain side effects and disease symptoms.
One of the most significant barriers preventing the further engagement of patients from an HTA standpoint is a lack of patient education. Patients need to be educated on the concepts underpinning HTA for them to understand how to contribute evidence that adds value to the process. Furthermore, if not adequately trained, patients may lack the confidence to voice their opinion in the presence of HTA decision-makers and clinicians, even when given the opportunity. As an example, a survey conducted in Italy in 2016 found that the requirement for citizens and patients to be trained was an impediment to establishing a more consistent approach to involving patients in the HTA process[2].
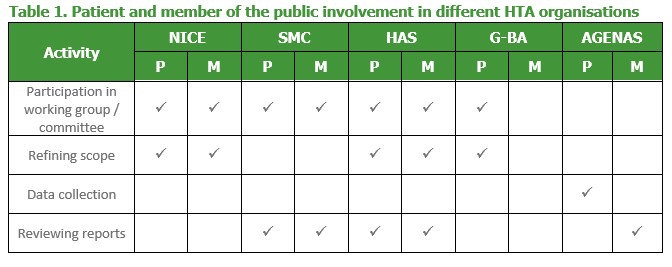
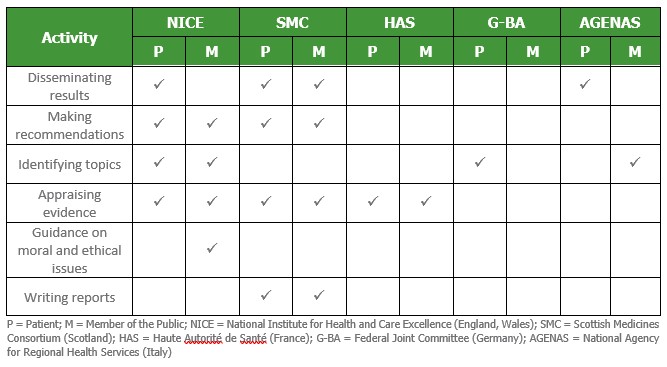
There is evidence that HTA organisations are increasing the involvement of patients, members of the public, or both in their processes. Table 1 indicates the current status across the EU5, based on a survey of international agencies[3]. Many HTA bodies are evaluating the impact of their patient and public involvement activities, with an ongoing commitment to starting or continuing such evaluations in future evident from the SMC, NICE, HAS, ZonMW and SBU3.
What is EUPATI?
The European Patients’ Academy on Therapeutic Innovation (EUPATI), funded through the Innovative Medicines Initiative (IMI) as a public private partnership, was a five-year multi-stakeholder project initially planned to run from February 2012 to January 2017. Comprising 30 bodies including patient organisations, academia, non-governmental organisations and industry, the objectives of the initiative were to build competencies and expert capacity among patients and the health-interested public by:
- Developing and disseminating education material for patients on the processes of research and development (R&D), clinical trials, personalised medicine, efficacy and safety assessments, risk-benefit assessments, health economics, and HTA;
- Increasing the capacity of ‘patient experts’ and well-informed patients in patient organisations to be effective advocates and advisors in medicines R&D, and;
- Empowering patients to provide appropriate patient-relevant advice and insight to industry, academia, authorities and ethics committees.
What has EUPATI achieved?
EUPATI has been described by the project coordinators as a ‘game-changer’ in the empowerment of patients and is a key driver of the public debate on patient involvement in R&D in Europe. EUPATI developed education materials targeted at two different levels:
- Expert Training Courses in English to patient experts, patient ambassadors and patient journalists, covering HTA principles and practices, as well as 5 other key areas. Two such courses were conducted in 2015/16 over a period of 14 months each, comprising over 150 hours of eLearning and two four-day face-to-face meetings
- An Educational Toolbox covering much of the same content as the expert training course, provided in 7 different languages and delivered primarily through presentations, videos and webinars. Though originally targeted at 12,000 patient advocates, by January 2017 over 100,000 individual users had accessed the Toolbox. These users were not limited to Europe, with almost 10,000 visitors logging on from Asia, and 2,700 from Africa.
EUPATI also established national platforms in 18 countries based on its model, and published guidance documents on patient involvement across the entire process of medicines R&D with regulatory agencies, HTA bodies, ethics committees and the pharmaceutical industry.
A significant impact was seen from the Expert Training Course across a range of areas, as evidenced by a survey of the 96 participants following their graduation. From an HTA perspective, the proportion of these course participants advising HTA / reimbursement agencies doubled because of the course, from 4% to 8%.
What does the future hold for EUPATI and patient involvement in HTA?
Based on its success, the decision was made to sustain EUPATI beyond its initial completion date of 2017, maintained on 25% of its previous budget. The Expert Training Course and Educational Toolbox will be the focus as the core EUPATI products, with content updated to keep pace with external developments as necessary. In addition, several areas have been identified for future development, including widening the training audience to include other stakeholders such as patients and carers at large, healthcare professionals, certain industry staff and policy makers, and geographical expansion into the USA.
The continuing work of EUPATI is therefore set to support the ongoing trend to increased patient involvement in HTAs, by educating a wider range of stakeholders, both in the EU and potentially further afield. It will be interesting to observe how patient involvement in HTA processes develops and whether this involvement will have a meaningful impact on the final decisions and greater access to medicines.
[1] Scott A M, et al. Patient advocate perspectives on involvement in HTA: an international snapshot. Scott and Wale Research Involvement and Engagement (2017) 3:2
[2] Moccia F. How to involve citizens and patients in HTA: the experience of Cittadinanzattiva. 3rd Global Forum on Medical Devices (2017)
[3] Weeks L, et al. Evaluation of patient and public involvement initiatives in health technology assessment: A survey of international agencies. Int J Technol Assess Health Care; 33(6); 715-723 (2017)
