What are the pro’s and cons of seeking NICE approval using the Medical Technologies Evaluation Programme?
Introduction
In England, market access for innovative medical devices is a less structured pathway than it is for pharmaceuticals. In most instances, it is the manufacturer’s prerogative to decide which access route is most suitable for their device and diagnostic. If they choose to, manufacturers can follow a local or regional Clinical Commissioning Group (CCG) driven approach, where their product is adopted incrementally on an individual CCG-by-CCG basis. While not a mandatory requirement, it is possible for the manufacturer to put forward their device for NICE assessment. Alternatively, physicians or CCGs can request a NICE evaluation, or it can be identified proactively by NICE themselves. This article will look at why manufacturers may choose to submit their product rather than solely pursuing a local, CCG-driven approach.
NICE appraisal approach
The National Institute for Health and Care Excellence (NICE) has several different programs, depending on the type of technology to be assessed (i.e. interventional procedures, diagnostics). For the purpose of this article, we will only focus on the Medical Technologies Evaluation Programme (MTEP), which assesses CE-marked medical devices (or if it is expected within one year)
The MTEP assesses medical devices believed to have tangible additional clinical benefits for patients at a similar cost as current standard practice (or alternatively, the same level of clinical benefit at a lower cost). Over a period of ~38 weeks, medical devices are assessed on both their clinical effectiveness and cost-effectiveness. Where other programmes carry out economic analysis through cost-utility analysis, the MTEP is different in that it carries out cost-consequence analysis. Rather than a single outcome measure being generated for all technologies (e.g. the QALY), the MTEP produces a benefit that is associated with the medical device (e.g. avoidance of hospitalisation)
Based on whether the Medical Technologies Evaluation (MTE) Committee believe the medical device provides sufficient additional benefits to patients and the NHS (e.g. reduced usage of resources or staff time), the Committee can make several different ‘case for adoption’ recommendations:
- Recommendation for use
- Recommendation for use in specific circumstances,
- Recommendation for use in specific circumstances and for development of further evidence
- Recommendation for use in a research context
- Case for adoption not supported
It is important to note, however, that the recommendations of the Committee are not mandatory for CCGs to implement, and there is no funding associated with the Committee’s guidance. Therefore NICE-recommended medical devices are only reimbursed when individual CCGs (or groups of CCGs) choose to procure the product. However, the MTEP will produce guidance for the adoption of the medical device within the relevant area of the NHS, as well as provide support through an adoption and impact team, which is obviously advantageous for the manufacturer.
The primary reason for seeking NICE approval is that it can be a major driver in product uptake. One of the main aims of the MTEP is to “promote faster uptake of new medical technologies in the NHS”, with guidance explicitly stating that “medical technologies are likely to be adopted more consistently and more rapidly if NICE develops guidance on them”. Ultimately, a NICE recommendation gives commissioners and healthcare providers confidence that the medical device is providing overall benefit to the patients and the NHS, especially if the medical device is eventually incorporated within a clinical guideline.
While the theory behind rapid uptake linked to a NICE recommendation is sound, there have been few studies that have looked at the direct impact of an MTEP (or Technology Appraisal) recommendation on the uptake of medical devices. This is primarily due to the challenge of collecting high-quality data around the use of medical devices in the NHS, but also because it is hard to verify whether the uptake of a new and innovative product is tied specifically to the NICE recommendation. For example, Leng et al. (2018) looked at the impact on the publishing of NICE guidance (from Technology Appraisals) on the uptake of three separate medical devices between 2000 and 20171. While some of the adoption graphs are dramatic (Fig.1), there was an acceptance that there are other factors which drive adoption of new medical devices (e.g. NHS structure, product competition) other than NICE recommendations.
Figure 1 – Uptake of liquid-based cytology following 2003 Technology Appraisal
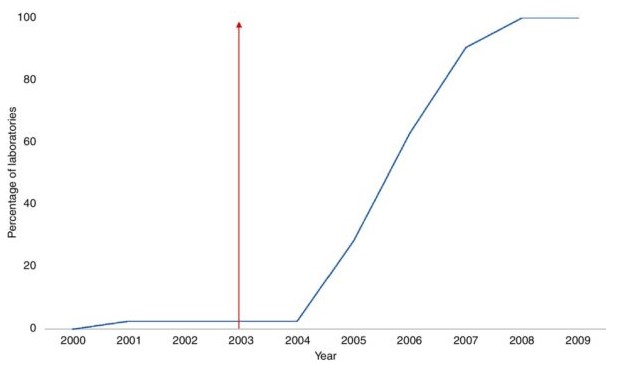
To undergo a NICE appraisal of any kind, however, there needs to be some level of clinical and/or economic evidence available to the appraisal committees. While the level of evidence is not comparable to that required for pharmaceuticals in the STA, and the MTEP are willing to accept evidence from a broader range of data sources (i.e. not RCT data), the level of evidence required may still be beyond the capability of most small manufacturers The risk with submitting medical device for appraisal with very limited evidence is obvious. Should NICE produce negative guidance for the device, then it is likely to impact adoption and uptake (at least until future evidence is generated).
Other than to drive rapid uptake, the secondary aim of the Programme is “to encourage collaborative research, in both industry and the NHS, to generate evidence on the clinical utility and/or healthcare system benefits of selected technologies”. As part of their final judgement, the MTEP can partially support the case for adoption and recommend the development of further evidence. This means that, alongside the MTEP, NICE work with the manufacturer to facilitate additional UK-focused real-world evidence through the creation of partnerships between the manufacturer and relevant researchers. The advantage to manufacturers is that they receive assistance in generating this data (e.g. partnerships with hospitals) that could strengthen both a later NICE assessment and future adoption within the NHS.
NICE have developed the MedTech Early Technical Assessment (META) tool (for which Remap Consulting is a partner), which is designed to help manufacturers identify evidence gaps and better understand the value of their device. NICE hope that the use of this tool should aid manufacturers in deciding whether their current level of evidence is suitable for submission and mitigate the risk of a negative recommendation. This is a clear sign that NICE are trying to persuade manufacturers to submit their technologies for appraisal.
In conclusion, the major driver for seeking NICE approval for a medical device is a more rapid and consistent uptake, although it is hard to quantify the effect. It is hoped the continuing rollout of the NICE META tool will help to mitigate the risk of negative guidance, and hopefully persuade more companies to pursue a NICE assessment.
References: