Introduction to the EU Joint Clinical Assessment (JCA)
With EU regulation 2021/2282, the European Parliament aimed to substitute the parallel evaluations of clinical data conducted by multiple country-specific HTA bodies with a single harmonised relative effectiveness assessment1. Some joint assessments have already been done within the framework of the EUnetHTA Joint Actions, but the use of the results and joint clinical assessments has remained limited by the member states2. Whilst the European Medicines Agency (EMA) assesses whether the benefits of a medicine outweigh the risks, and country-specific HTA bodies determine added benefit and value, the JCA is intended to be a streamlined process in which a single submission of data addresses issues of relative effectiveness and relative safety. However, the JCA will not make value judgements or conclusions on added clinical value and is non-binding. The ability to make value judgements and pricing and reimbursement decisions remain under the sovereignty of the member states. Although the results of the JCA are non-binding, member states must give ‘due consideration’ to the JCA report, which means that the report should be part of the documentation that they assess during decision making. Member states may choose to use to utilise the data in different ways, such as using JCA to inform pricing and reimbursement decisions without needing extensive additional evidence, or requesting additional clinical analysis requiring further evidence (e.g., additional patient groups or comparators). Countries such as Australia have already begun to investigate how to incorporate the EU HTA and JCA into their own national processes3. The result of the potential variance in the use of JCA data will ultimately have different effects on manufacturers.
What is the JCA process?
The process for the joint clinical assessment is overseen by the Member State Coordination Group on HTA, which appoints both an assessor and co-assessor from different member states who are responsible for the clinical assessment, drafting of a report and consultation with stakeholders4. Following approval by the Coordination Group, the reports are consequently published by the European Commission. A top-line breakdown for the JCA divides the process into four steps:
- The scoping phase, encompassing the development and validation of PICOs
- JCA dossier development phase
- JCA dossier assignment phase
- The publishing of the final JCA report
Although the length of the scoping phase has not yet been concretely established5, timelines for JCA dossier development are ~6 months, and development of the dossier by the manufacturer ideally should be started at the time of EMA marketing authorisation (MA) submission, to allow for sufficient preparation. The JCA dossier is required 45 days prior to the EMA’s Committee for Medicinal Products for Human Use (CHMP) opinion and will be published at the latest 30 days after approval of the medicine by the EMA. As a result, the JCA report will be amongst the first assessments globally, which may have a resultant effect on country-specific HTA processes and procedures to start pricing negotiations.
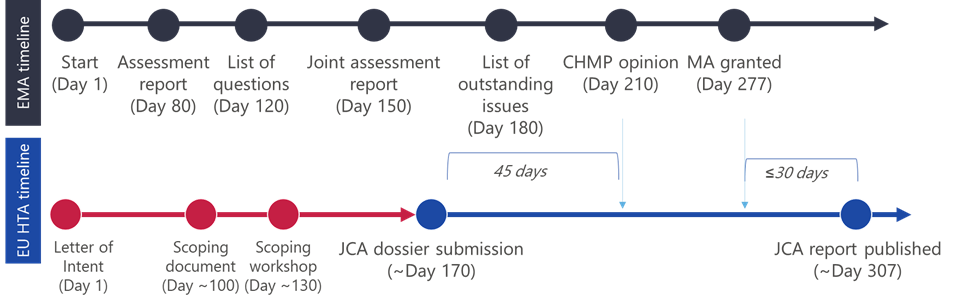
The scoping process and PICO consolidation
Prior to the submission of the dossier, a scoping process is conducted, and the results of this process comprise the research question and assessment scope for the dossier. The scoping process aims to define the PICOs (Population, Intervention, Comparator, Outcomes), which have the role of specifying the framework for assessment and the data requirements from the manufacturer 4. The PICO survey is sent to all EU27 countries, which provide information about their needs in terms of the PICO parameters. The returned quantity of PICOs could be very high based on different needs in different member states6, resulting in the need for consolidation of the PICOs by the JCA assessors in the Committee for Scientific Consistency and Quality (CSCQ) JCA meeting4. This should result in a limited number of PICOs which form the basis of the JCA dossier. More information on the scoping process and PICO consolidation can be found in our article here.
The JCA dossier and submission process
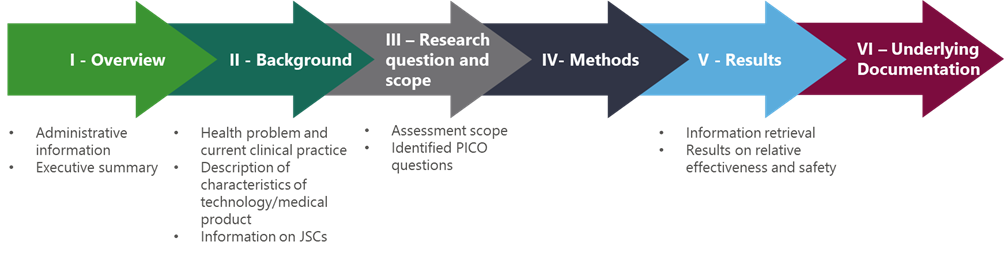
After PICO consolidation, the company will have to develop and submit the dossier based on the scope defining the data request for assessment. This dossier is broadly split into 6 parts which inform the outcome of the assessment7:
- Overview
- Background
- Research question and scope
- Methods
- Results
- Underlying documentation.
The first overview section encompasses administrative information on the manufacturer responsible for submission and an executive summary of the dossier content, with particular focus on the assessment scope and response to PICOs. Following this, as with country-specific HTAs, the background includes the characterisation of the health condition to be treated, characterisation of the target population, the current clinical pathway for the health condition including significant variations between countries, characterisation of the medical technology in question, and its regulatory status. At this point, any joint scientific consultations conducted under this assessment are also included. Of significance is the research question and assessment scope section, as this represents the decision problem from different healthcare systems and will be the section in which the manufacturer must address the PICO questions with evidence or justify why the PICO question cannot be addressed. Here, separate PICO questions should be presented separately rather than as an amalgamation. Following this, the methods section includes both the criteria for selecting studies for joint clinical assessment and information retrieval, so as to provide evidence for the submission. The results section includes the studies used in the submission dossier on relative effectiveness and relative safety, and results for individual PICO questions based on patient population, patient characteristics, and outcomes for the PICO. The dossier is then concluded with the underlying documentation for the submission dossier. This document structure is summarised below.
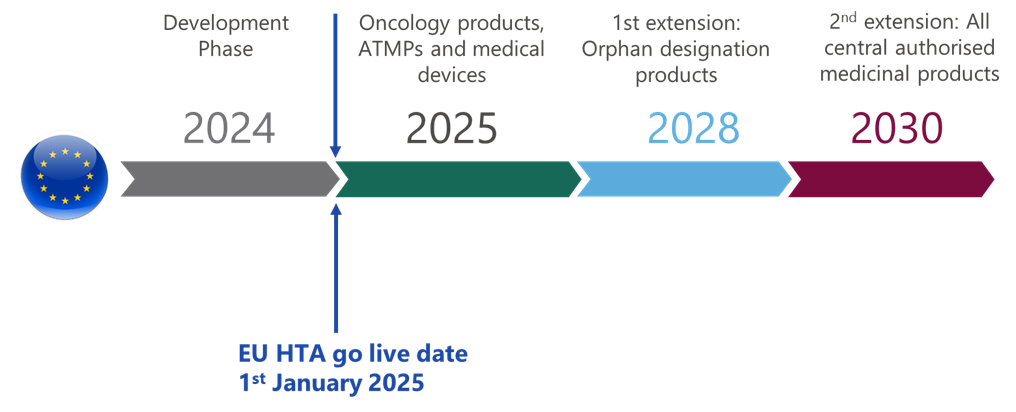
Current progress and future developments
As it stands, the EU HTA is projected to go live on January 1st 2025. During 2025, oncology products, ATMPs and medicinal devices will come under the remit of the JCA, orphan designation products by 2028, and all central authorised medicinal products by 20308, meaning the implementation of the joint clinical assessment is soon to come.
In preparation for this, EUnetHTA is progressively releasing templates and guidelines to facilitate the assessment. The most recent guideline releases include procedural guidelines for appointing assessors, interactions between manufacturers and HTA bodies, for the JCA submission dossier, and for the production of a JCA on medicinal products and devices. EUnetHTA has also released a template for the JCA report, showing ongoing progress in the development of resources9. The only development that remains to be made is further guidance for the JCA by the Consortium Executive Board (CEB), which will include a submission dossier template8. The release of this document will mark the completion of preparation documents for the launch of the JCA, according to the information available from EUnetHTA 21 9. However, there remain concerns and challenges that must be addressed for successful implementation of the JCA. For example, there are concerns that EUnetHTA 21 has not included provisions to address specificities of medical technologies, or sufficient detail on adapted methods that may be needed for assessing orphan drugs and ATMPs10,11. This, combined with views that current methods are not harmonised and seem an amalgamation of national practices, suggest to some that there will be no decrease in administrative burden11. To successfully implement the joint clinical assessment, these challenges must be addressed.
Conclusion
To conclude, in preparation for implementation in 2025, the joint clinical assessment is ongoing in its development, and EUnetHTA 21 is developing guidance and templates to facilitate this. In tandem with JSCs, the JCA is aimed to speed up access to new therapies, reduce duplication of work and harmonise methodologies used for clinical evaluation with different health care agencies within the EU. Although much has been released on how the submission process works and what this entails, implementation will not be straightforward as there remain concerns and clarification on areas such as how individual countries will use the JCA reports, the level of duplication that may occur, and additional level of evidence requirements for specific countries. It will be interesting to see how countries use JCA data to inform their own HTA processes and how successful the JCA ends up being in harmonising the health technology assessment process.
Sources:
- Joint clinical assessment in the EU: Pan-European HTA for drugs and medical devices will become reality. Xcenda. https://www.xcenda.com/insights/htaq-spring-2022-joint-clinical-assessment-eu. Accessed 23rd March 2023.
- Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. EUR-Lex. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R2282. Accessed 23rd March 2023.
- HTAR (HTA-Regulation): Implementation in Austria. Austrian Institute for Health Technology Assessment GmbH. https://aihta.at/page/htar-hta-verordnung-implementierung-in-oesterreich/en. Accessed 23rd March 2023.
- D4.2 Practical Guideline on Scoping Process. EUnetHTA. https://www.eunethta.eu/wp-content/uploads/2022/09/EUnetHTA-21-D4.2-practical-guideline-on-scoping-process-v1.0.pdf. Accessed 23rd March 2023.
- The Joint Clinical Assessment (JCA) and its implication for the industry. Pharos. https://pharos-healthcare-consulting.com/news/the-joint-clinical-assessment-jca-and-its-implication-for-the-industry/. Accessed 23rd March 2023.
- EU Joint HTA: How will EUNetHTA balance contrasting decision drivers across Europe with the desire to produce a single Joint Clinical Assessment to be applicable in all member states? Remap Consulting. https://remapconsulting.com/hta/eu-joint-hta-how-will-eunethta-balance-contrasting-decision-drivers-across-europe-with-the-desire-to-produce-a-single-joint-clinical-assessment-to-be-applicable-in-all-member-states/. Accessed 23rd March 2023.
- D5.1 Submission Dossier Guidance. EUnetHTA. https://www.eunethta.eu/wp-content/uploads/2022/12/EUnetHTA-21-D5.1-Submission-Dossier-Guidance-v1.0.pdf. Accessed 23rd March 2023.
- EUnetHTA 21 – Stakeholder Kick Off Meeting. EUnetHTA. https://www.eunethta.eu/wp-content/uploads/2021/12/EUnetHTA-21-Stakeholder-Meeting-03.12.-FOR-WEBSITE.pdf. Accessed 23rd March 2023.
- Joint HTA Work. EUnetHTA. https://www.eunethta.eu/jointhtawork/. Accessed 23rd March 2023.
- EU Regulation on Health Technology Assessment (HTA). MedTech Europe. https://www.medtecheurope.org/news-and-events/press/eu-regulation-on-health-technology-assessment-hta/. Accessed 23rd March 2023.
- Proposals On EU-Wide Joint Clinical Assessments Fall Short, Warn German Industry Groups. Pink Sheet Pharma Intelligence. https://pink.pharmaintelligence.informa.com/PS147635/Proposals-On-EUWide-Joint-Clinical-Assessments-Fall-Short-Warn-German-Industry-Groups. Accessed 23rd March 2023.
