Italy is one of the most prominent users of Managed Entry Agreements (MEAs) for the regulation of access to new treatments that are either high in cost or come with uncertainties over their clinical benefit and/or economic value.
This week AIFA’s Medicines Utilisation Monitoring Centre (OsMed) has published its annual national report “Medicines use in Italy” for the year 2022.
According to the report, the adoption of managed entry agreements resulted in 195.2 million of savings for the healthcare system in 2022.
Italy uses several patient-level MEAs, both outcomes-based agreements and financial based-agreements.
Outcomes-based agreements are generally used when uncertainty exists over a product’s clinical benefit and include:
- Risk-sharing: in the event of treatment failure, the cost is shared between the SSN (national health service) and the manufacturer
- Payment by Result (PbR): in the event of treatment failure, the manufacturer covers the entire cost, refunding the full treatment cost for non-responders. There are two variants of PbR:
- Success fee: the manufacturer is paid by the SSN only if clinical outcomes are achieved
- Payment at result (PaR):the SSN makes staggered payments to the manufacturer based on outcomes achieved at specified timepoints
Financial-based agreements are used when the uncertainty is around the product’s economic impact and include:
- Cost sharing: portion of the cost is reimbursed by the manufacturer regardless of clinical outcomes
- Capping: an expenditure ceiling per patient is set. If exceeded, the manufacturer reimburses the SSN for the additional costs
At the end of 2022, 50% of the total managed entry agreements in place were outcomes-based agreements. Of these, 76% were payment by result and 23% were payment at results. The remaining 50% were financial-based agreement and of these, 46% were cost-sharing and 53% were capping agreements.
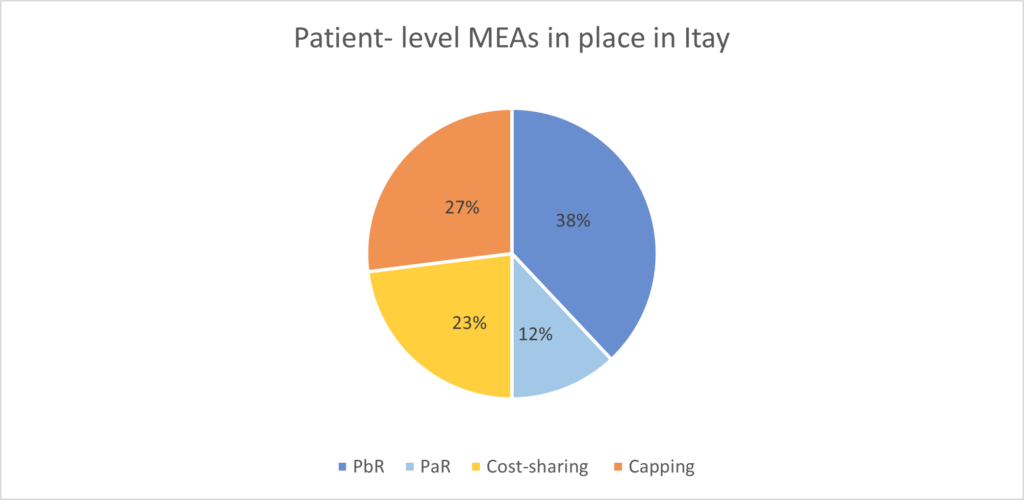
Italy also adopts two types of population-level MEAs:
- Expenditure ceilings per product: a spending limit is set for a drug in its first 12 or 24 months post-launch. Beyond this limit, the remaining costs are paid back by the pharmaceutical company to the regions. In 2022, 12 drugs were subject to an expenditure ceiling agreement.
- Price-volume agreements: incremental discounts on list price are provided based on the volume. These can be in the form of a price reduction or of payback to the regions.
In 2022, 4 drugs were under a price-volume agreement.
In 2022, the implementation of these MEAs led to a cost saving of 195.2 million with 73% of the total savings attributed to expenditure ceiling and cost-sharing agreements.
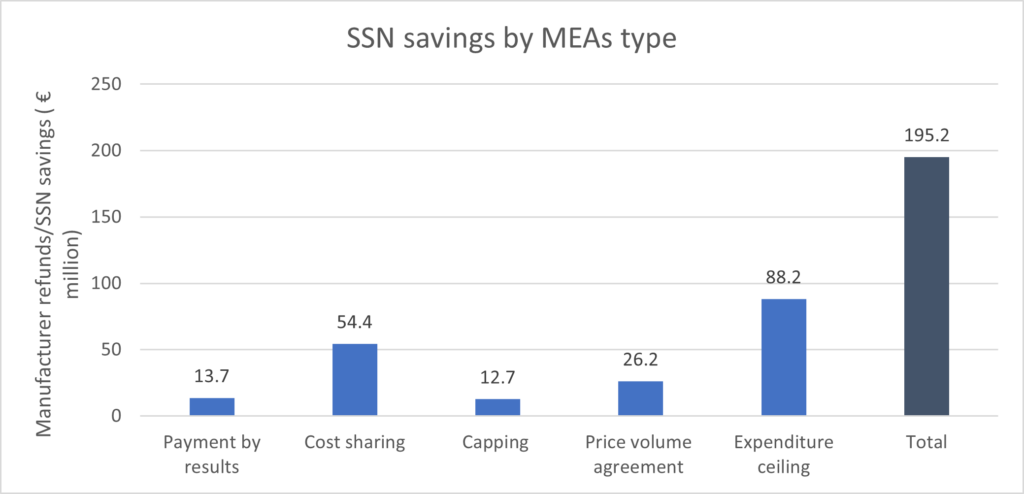
Source:
