Over the past few months, NICE have proposed a number of changes that would impact the market access landscape in England and Wales. These include the introduction of a ‘fast track’ assessment process, abbreviated health technology appraisal processes and the consideration of budget impact assessments within technology appraisals. NICE are also considering charging manufacturers for the cost of technology appraisals, which is a significant departure from the current situation.
NICE to charge for technology appraisals
NICE have proposed to charge manufacturers £99,000 to £142,000 for the evaluation of a single technology appraisal (STA), from April 2017. Complex multiple technology appraisals could cost up to £282,000 (see below). These fees will be the highest health technology assessment (HTA) fees charged across the world and are significantly higher than fees charged by other countries, (for example Australia’s PBAC’s ~£65,000).
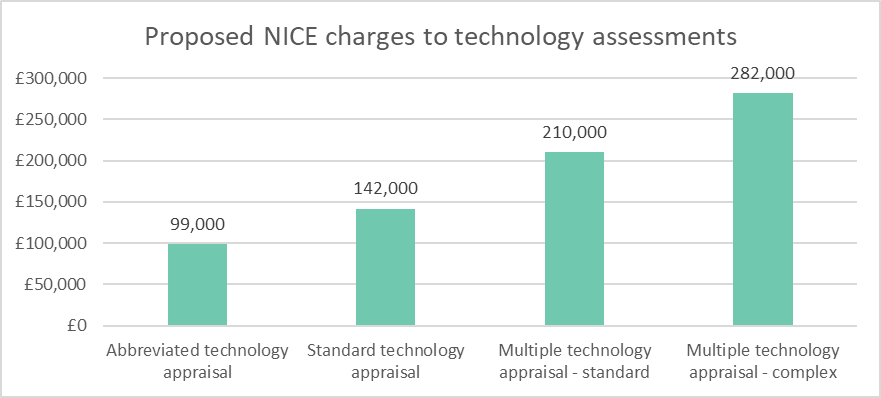
The fees will be the same for all companies irrespective of size, with the only concession being smaller companies can pay in instalments. Should the company refuse to pay, then NICE will terminate the appraisal and issue a ‘NICE is unable to make a recommendation because the manufacturer has not provided a submission’ statement. Unsurprisingly, the Association of the British Pharmaceutical Industry (ABPI) says that they won’t accept fees without reform of NICE to ensure the appraisal process becomes more efficient. It also argues that such a fee-based process has the potential to create further inequalities in access to medicines for NHS patients.
NICE consider introducing abbreviated technology appraisals
As a result of the increasing number of technology appraisals undertaken, NICE have proposed to introduce an abbreviated technology appraisal process (ATA). The ATA will be applicable to products that are expected to provide similar or greater health benefits, at a similar or lower cost, to those that have been previously recommended by NICE for the same indication. The methods for the ATA will follow the existing NICE methods of appraisal except for the economic evaluation, where NICE would accept a cost-comparison analysis. This would mean that a cost-utility analysis is not essential.
The level of clinical evidence considered would be similar to the current assessment process and will include a full systematic review. NICE are proposing two outcomes to the ATA:
1. Technology provides similar or greater benefits at a similar or lower cost than the comparator(s). Recommended ‘as an option’
2. Technology provides less health benefit at a similar or greater cost or Technology provides similar health benefits at a greater cost. Not recommended
NICE to include budget impact within NICE assessments for products with high budget impact
High drug prices, such as for Gilead’s hepatitis C treatment Sovaldi, raised concerns from NHS England over their ability to fund products despite NICE considering them to be cost-effective. NICE are considering including a £20 million budget impact threshold in their assessments. If annual sales are expected to exceed £20 million within the first three years of launch, manufacturers would be required to put a commercial agreement in place with NHS England (the budget holder). This is in order to avoid compromising access to other forms of care. In addition, there will be more flexibility regarding the time (currently set at 3 months) available to implement any new NICE guidance for products exceeding the £20 million threshold.
NICE to approve products with a cost-effectiveness threshold of less than £10,000
NICE are proposing introducing a new ‘fast track’ assessment process. This is aimed to improve patient access to medicines for drugs which are considered to be cost effective with an ICER of less than £10,000. The new ‘fast track’ would mean NICE would produce ‘light touch’ decisions. NICE estimate that the ‘fast track’ process would save 25% in process time compared with standard appraisals, with final guidance issued up to 3 months earlier than normal. Whilst this sounds appealing, it will only apply to ~15% of products.
These changes could be viewed as NICE trying to take a more pragmatic approach to HTAs. NICE have realised that there are a number of instances where a full STA is not required. Walking away from full STAs in these instances could save resources not only for NICE but also for the manufacturer. The assessment of budget impact is unlikely to impact most manufacturers. However, charging a fees for conducting STA will not be welcomed by manufacturers, but is a reflection of the effort involved in producing an STA. As such, these changes represent an evolution of NICE, which should bring benefits to some manufacturers, particularly those whose products are less innovative.
