Introduction
Before the EU joint HTA comes into force in 2025 there has already been a system for pan-European cooperation in the payer scientific advice process in the form of parallel joint scientific consultations (JSCs) and multi-HTA early dialogues. JSC’s allow pharmaceutical developers to gain feedback and advice from regulators and HTA bodies on their development plans to support obtaining marketing authorisations and positive pricing and reimbursement outcomes. This non-binding scientific advice is given before the start of pivotal clinical trials (after a feasibility study) in order to improve the quality and relevance of data produced by RCTs in preparation for future HTA assessments or reassessments.
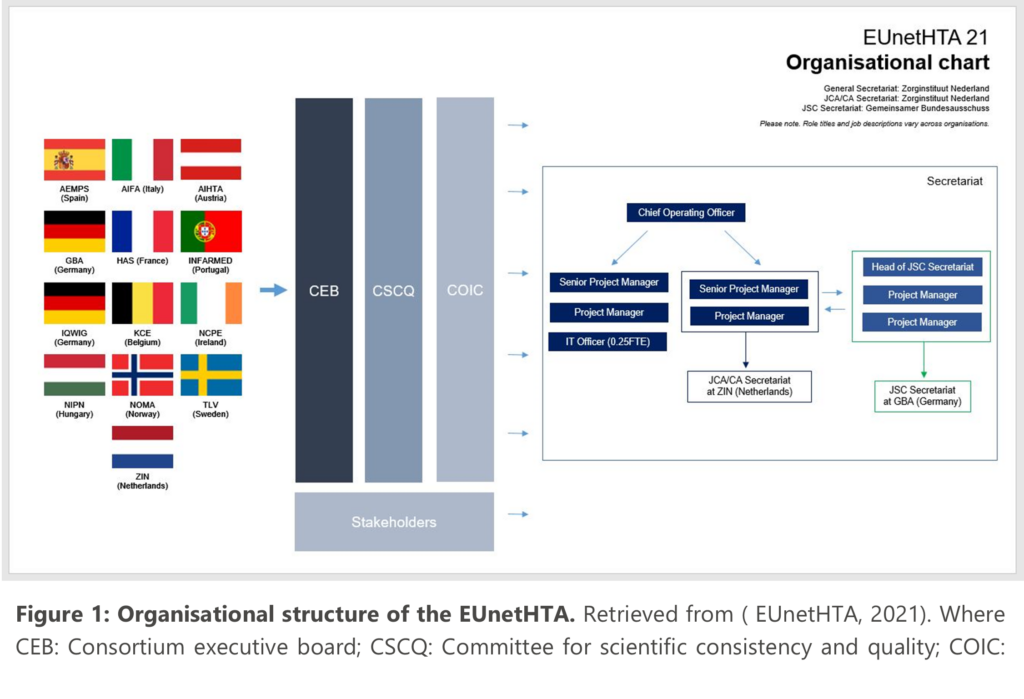
These have been run in parallel by the European Medicines Agency (EMA) and the European Network for Health Technology Assessment (EUnetHTA) since September 2017, with the current contract running from September 2021 through until September 2023. Although parallel consultations bring significant benefits they can be run without the EMA if that is desired. These JSC’s are carried out by members of the Committee for Scientific Consistency and Quality (CSCQ), which is responsible for ensuring the scientific consistency and quality of EUnetHTA’s outputs. The CSCQ is a division of EUnetHTA and made up of the different HTA organisation members:
What is the current JSC and how does it enable market access success?
Consultations allow for dialogue between an applicant and HTA agencies at an early stage in the development process, allowing for integration of the different requirements for study design and economic evidence generation. Conversations could centre around clinical trial design, choice of comparators, endpoints, interventions, relevant outcomes, patient populations, quality of life and patient groups for pivotal trials and post-launch evidence generation.
The number of available slots on this programme was limited, with a maximum of 8 JSCs, and as of the 31st August last year the 2nd open call for JSCs has closed. The CSCQ has reviewed and selected the 5 additional JSCs to be conducted within the framework of EUnetHTA 21. Amongst the selected JSCs there are three oncological and two non-oncological products:
- 5 First in class
- 2 Orphan designations
- 2 Advanced therapy medicinal products
- 1 Priority medicines
- 1 Micro, small and medium sized enterprise
What are the selection criteria and timelines for current JSCs?
Each JSC procedure takes approximately 4.5 months and begins when a draft briefing package is submitted, which is followed by a face-to-face meeting and finally the EUnetHTA’s final written recommendations. To obtain a JSC an application form must be completed, and the selection of products is based on the 6 criteria listed below:
Unmet medical needs
At the time of the application for a JSC there must be no viable method for diagnosis, prevention or treatment especially concerning rare, life-threatening or chronically debilitating diseases. Along with an application, companies should outline available diagnostic, prevention or treatment options, the efficacy of such methods and how the unmet need is not addressed by the current options.
First in class
The drug must be the first representation of the class of substance for the disease in question and overall, the product has to be an innovative technology.
Potential impact on patients, public health, or healthcare systems
The pharmaceutical in question must lead to significant improvements in patient morbidity, mortality and quality of life with a good side effects profile. Preliminary data on patient relevant outcomes should be provided if it is available.
Significant cross-border dimension
For the disease to have a serious cross border impact it must be evenly distributed across Europe or at least equivalent in 3 or more countries and provide a serious risk to cross border health.
Major Union-wide added value
For a product to have major union wide added value, the clinical evidence should have been collected in Europe or at least adequately reflect the patient population and European health standards. The manufacturer needs to show patient-relevant outcomes and the product should not just be an addition to the portfolio of existing therapeutic options.
Union clinical research priorities
Rationale must be provided if the disease to be treated by the product is in the focus of EU clinical research and in general the union’s priorities focus on relevance to the European population. Examples of programmes are:
- EU4Health Programme
- Europe’s Beating Cancer Plan
- Horizon Europe
- Innovative Health Initiative
Post 2025 and the implications for companies?
One of the supplementary aims of the EUnetHTA 21 initiative is to find common positions for the HTA requirements of its members, thereby preparing for the implementation of the regulation on HTA in 2025. Currently, a member state will inform the EUnetHTA 21 when it carries out a national scientific consultation (NSC) on a product that has been the subject of a JSC. This national consultation should complement the JSC by addressing context specific issues related to the national HTA system. Post January 2025 duplicated full scientific consultations at the national level will conclude as joint HTAs will come into force thereby removing the need for NSCs. Clarifications on national positions are exempt but should not contradict agreed on positions from the JSC.
For manufacturers of oncology, ATMPs and orphan drugs, there is a need to act now to consider the potential EU HTA opportunities, JSCs will be a valuable resource for HTA submissions post 2025 and therefore companies need to be planning for this now. Manufacturers should be considering the JSC selection criteria when collecting early phase clinical data so that they have the best possible chance of being selected for the limited number of slots. Companies should also think about prioritising products that will benefit the most from JSCs and develop some early argumentation on why they should be included on the programme, again based on the selection criteria.
Some of the necessary documents for JSC have already been developed and should be reviewed for further insight into the process post 2025. These include:
Application form:
Formally requests a parallel JSC by the EUnetHTA and EMA.
Briefing document template
Sets out the key characteristics of the product in question including method of delivery, dosing, etc, as well as the reasons for wanting a JSC. During the JSC process this document is also used to frame the questions and queries that the manufacturer would like the EUnetHTA/EMA to answer. This document will go through multiple iterations to address regulator’s comments.
The current versions of the briefing document template and applications forms can be found on the EUnetHTA 21 website.
Sources:
- EUnetHTA. (2021, September 17). About EUnetHTA 21. Retrieved from EUnetHTA: https://www.eunethta.eu/about-eunethta/
- EUnetHTA. (2020, July 3). Early Dialogues. Retrieved from EUnetHTA: https://www.eunethta.eu/ja3services/early-dialogues/#:~:text=EUnetHTA%20defines%20an%20Early%20Dialogue,HTA%20assessment%20%2F%20re%2Dassessment
- EUnetHTA. (2021, September 17). Frequently asked questions (FAQs) regarding EUnetHTA 21 Joint Scientific Consultations (JSC). Retrieved from EUnetHTA: https://www.eunethta.eu/jscfaq/
- EUnetHTA. (2021, September 2021). Parallel EMA/EUnetHTA 21 Joint Scientific Consultations (JSC). Retrieved from EUnetHTA: https://www.eunethta.eu/jointhtawork/parallel-consultation/
- EUnetHTA. (2022, August 31). 2nd Open Call for Parrallel EMA/EUnetHTA 21 Joint Scientific Consultations (JSC). Retrieved from EUnetHTA: https://www.eunethta.eu/wp-content/uploads/2022/06/EUnetHTA-21-JSC_2nd-Open-Call-Text_June-August.pdf
- EUnetHTA. (2022, August 31). Joint Scientific Consultations (JSC). Retrieved from EUnetHTA: https://www.eunethta.eu/jsc/
- EUnetHTA. (2022, June 10). Parallel EMA/EUnetHTA 21 Joint Scientific Consultation (JSC) Application Form EUnetHTA 21 2nd Open Call (6 June, 2022 to 31 August, 2022). Retrieved from EUnetHTA: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.eunethta.eu%2Fwp-content%2Fuploads%2F2022%2F08%2FEUnetHTA-21-JSC_Application-Form_2nd-OpenCall_June-August.docx&wdOrigin=BROWSELINK
- EUnetHTA. (2022, September 28). Parallel EMA/EUnetHTA 21 Joint Scientific Consultation Briefing document template. Retrieved from EUnetHTA: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.eunethta.eu%2Fwp-content%2Fuploads%2F2022%2F10%2FJSC_EUnetHTA-21_Briefing-Document_Template_28092022_final.docx&wdOrigin=BROWSELINK
- Europ[ean medicines Agency. (2022, November 16). Parallel joint scientific consultation with regulators and health technology assessment bodies. Retrieved from European medicines Agency: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-advice-protocol-assistance/parallel-joint-scientific-consultation-regulators-health-technology-assessment-bodies
- European Parliament, Council of the European Union. (2021, December 15). Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. Retrieved from EUR-Lex: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32021R2282
- Miglierini, G. (2022, April 29). A new joint worknplan to 2023 fro EMA and EUnet21. Retrieved from European Industrial Pharmacists Group: https://eipg.eu/a-new-joint-work-plan-to-2023-for-ema-and-eunethta21/
- Salvatore, V., & Ragucci, G. (2022, August 25). The EU HTA regulation: a new frontier for access to innovative technologies. Retrieved from European Pharmaceutical Review: https://www.europeanpharmaceuticalreview.com/article/173762/the-eu-hta-regulation-a-new-frontier-for-access-to-innovative-technologies/