Joint Scientific Consultation
Strategic guidance for engaging with HTA bodies under the EU HTA Regulation

Strategic guidance for engaging with HTA bodies under the EU HTA Regulation

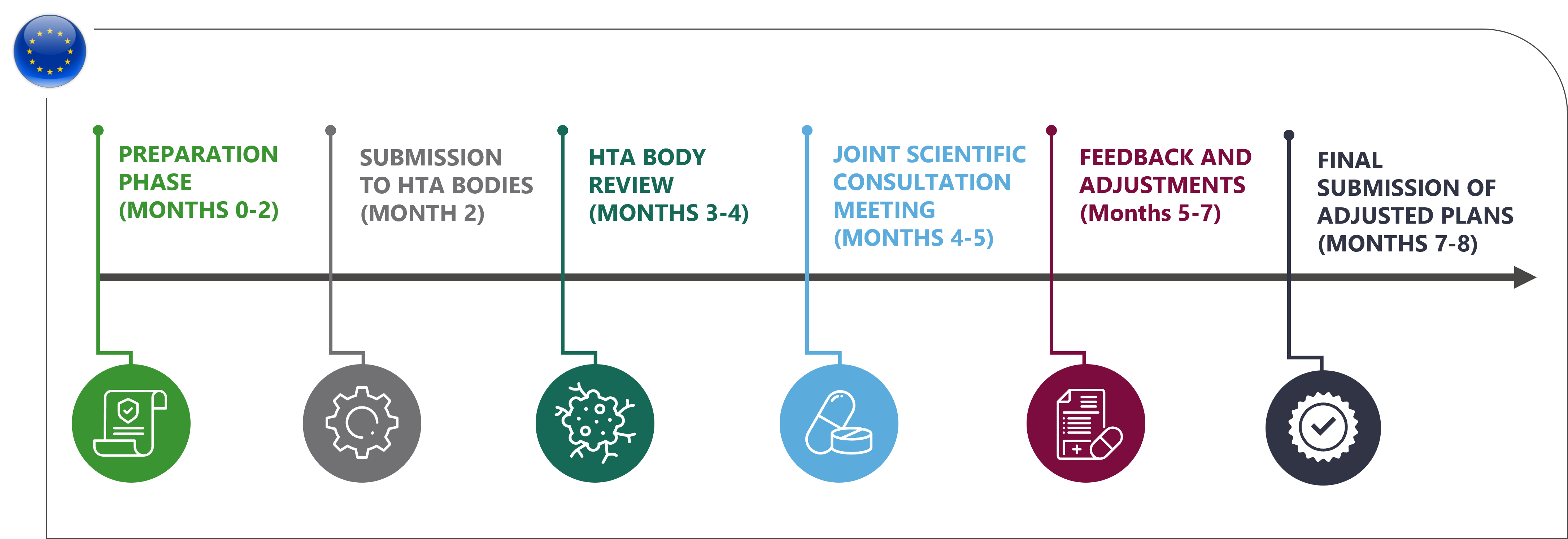
The launch of the EU HTA regulation in January 2025 marks a significant shift in how pharmaceutical and biotech companies will interact with Health Technology Assessment (HTA) bodies across Europe. One of the cornerstones of this regulation is the Joint Scientific Consultation (JSC), a collaborative process that allows manufacturers to receive critical early advice from multiple HTA bodies. This guidance ensures that clinical and economic evidence generation strategies align with the requirements of these HTA bodies, helping to mitigate risks and enhance the likelihood of successful market access.
Joint Scientific Consultations (JSC) are an integral part of the EU HTA Regulation, enabling developers to seek advice from multiple HTA bodies early in the development process. Through JSC, companies can gain clarity on the data and evidence needed to meet HTA requirements, improving the likelihood of favourable assessments during the Joint Clinical Assessment (JCA)
With extensive experience in market access and HTA strategies, our team at Remap Consulting is uniquely positioned to help you navigate the complexities of JSC. We provide strategic guidance and actionable insights to ensure your product development aligns with HTA expectations, ultimately enhancing your chances of success in the European market.
Engaging in a JSC is an essential step in reducing uncertainty and ensuring the success of your product in the European market. By obtaining early advice from HTA bodies, manufacturers can adjust their development plans to meet the diverse requirements of multiple HTA authorities, reducing the risk of delays or negative outcomes during the Joint Clinical Assessment (JCA) phase.
There is no excerpt because this is a protected post.
Read moreWe’re delighted to announce our participation at ISPOR Europe 2024 in Barcelona this November. We’ll…
Read moreOur latest article discusses key strategies for pharma manufacturers’ commercial readiness for EU HT…
Read moreWe summarise the key discussions from HTAi 2024, with a focus on sustainability, networks and innova…
Read moreWe discuss the updates on EU HTA from the June meeting of the Member State Coordination Group on Hea…
Read moreLearn how to navigate the upcoming EU HTA regulation and optimise market access strategies for oncol…
Read moreBack in January this year, we summarised the key trends our market access experts believed would dom…
Read moreWe summarise the World Orphan Drug Congress Europe 2023 including keynote and panel sessions on EU H…
Read moreOur research aims to gauge awareness of the new EU HTA process within the pharmaceutical industry in…
Read morePaul Craddy founded Remap Consulting in January 2014 to help clients optimise their pricing, health economics and market access outcomes.
Prior to that, Paul was Head of Diabetes for Pricing, Market Access and Health Economics for EUCAN within Takeda Pharmaceuticals. In this role, Paul was responsible for leading the franchise including managing the launch of a new diabetes therapy, providing input into R&D for pipeline products and optimising in-market products. Paul was also a member of the PMAHE management team, helping set the strategic direction of the PMAHE department.
During his time in Nycomed, Paul led the strategic pricing team responsible for global pricing decisions for Nycomed’s global brands. He was also a core member of the PMAHE integration team during the Takeda takeover, helping to design and implement the new PMAHE organisation within Takeda.
Preceding Nycomed, Paul was a PMA consultant with IMS Consulting based in Cambridge, UK. Here, he managed numerous global PMA projects across a variety of international clients, developing launch pricing strategies, creating payer value propositions & communications and managing market access reorganisations.
Paul holds a PhD in molecular biology from Edinburgh University and has a BSc Biology from Manchester University.
With over 20 years pharmaceutical experience, within both consultancy and pharmaceutical companies, Graham brings expertise in embedding market access drivers into clinical development programme; developing global product launch pricing strategies and producing HTA submissions to address payers’ pricing and reimbursement requirements.
Graham’s industry experience includes GSK, start-up biotech companies and Ferring pharmaceuticals. During his time at Ferring Graham was Global Head of Market Access and Pricing taking responsibility for all market access activities from incorporation of payer value into clinical development programs through to development and implementation of the market access and pricing strategy for global product launches.
Graham’s consultancy experience was obtained within both IMS consulting (previously Cambridge Pharma) and Adelphi Values whereby he was responsible for global pricing and reimbursement projects ranging from strategic insights to global pharmaceutical companies; market access training to senior management and affiliates; development of biosimilar access strategies and EU pricing and reimbursement submissions.
Graham has worked across many therapeutic areas including Oncology, Gastroenterology, Urology, Fertility, Obstetrics and Endocrinology.
Graham has a Ph.D. in neuropharmacology from Durham University and an MBA from Aston University. He currently lives in near Manchester, UK.