Introduction
The 2021 EU HTA Regulation and the recent March 2024 Draft Implementing Act highlighted one of the most critical shifts in European market access.1,2 The EU HTA consists of two main parts, Joint Clinical Assessments (JCA) and Joint Scientific Consultations (JSC), and is designed to promote the harmonisation of clinical assessments at the EU level. EUHTA has the primary goals of 1) reducing redundant HTA activities, 2) promoting efficiency in decision-making processes, and 3) reducing the time to access for patients. However, there is a tension here in that time to access for patients is often driven by price negotiations. This process will remain at the country level along with local HTAs, which are still occurring, therefore not reducing redundant HTA activities.
As with any large-scale process change, taking full advantage will be imperative for commercial success. Doing this will require reviewing internal structures and teams to ensure they are appropriate for the task at hand. This will involve compiling the necessary internal processes, ensuring resources are available and enabling cross-functional collaboration, which we will discuss in this article. We recently published a series of articles discussing organisational structures, what good looks like, and how to integrate market access strategy with evidence development successfully.
The paradigm changes in market access that the EU HTA represents require pharmaceutical manufacturers to dedicate significant resources to preparing for its implementation. This article aims to guide how to restructure market access organisations in response to the EU HTA and ensure commercial success.
Understanding the EU HTA: what will be changing and when?
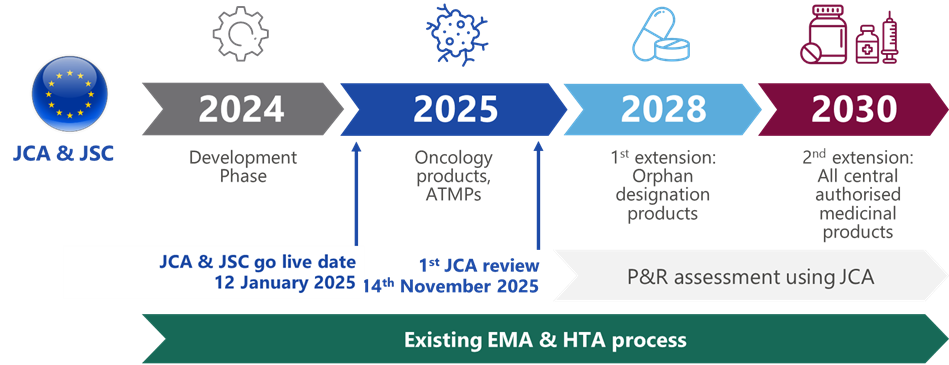
We have extensively covered the beginning of EU HTA in 2025 in articles and webinars, all accessible via our website. The implementation will begin with joint assessments of new oncology medicines and advanced therapy medicinal products (ATMPs) in January 2025 (Figure 1).1,3 This will be extended to orphan products in 2028 and finally to all new medicines in 2030, with interim reviews to assess the success of each stage.1,3
Although an exciting note and a point raised by Meindert Boysen, the former Head of International Affairs at NICE, is that JCA will begin with amongst the most complex health technologies from an HTA perspective. This is in direct opposition to the AUS-CAN-NZ-UK Collaboration Arrangement, which has prioritised collaboration on relatively straightforward submissions that are more suited to publishing HTA guidance closer to the time of marketing authorisation.4
Joint clinical assessments will be driven by assessors appointed from the EU HTA agencies by a member state coordination group on HTA and performed by two member state agencies in parallel.2 Considering this, it is likely that due to the current variety of HTA processes in place across the EU, there will be differences in how these assessments will be interpreted, depending on the appointed assessors. Therefore, any organisational changes manufacturers implement must be capable of adapting to the appointed assessors. From our perspective, these issues will challenge the objectives of increased efficiency and reduced redundancy.
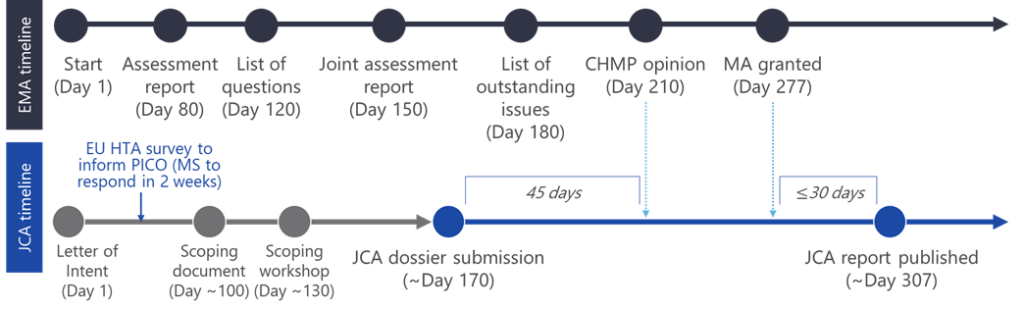
The JCA process will begin simultaneously with the European Medicines Agency (EMA) marketing authorisation submission and run in parallel, potentially placing significant strain on companies’ internal teams. This should be factored into how organisational structures may need to adapt (Figure 2). A further consideration in planning for the EU HTA is the tight timelines, where manufacturers will only have 100 days from PICO selection to the JCA dossier submission.
Vital organisational changes that will be needed
As previously mentioned, there are several challenges that the EU HTA poses that must be tackled, at least in some part, by changes in the way manufacturers are organised and undergo HTA submissions. We believe this preparation can be subdivided into three central tenants:

Well defined processes
The first step in preparing for the EU HTA will be establishing well-defined processes for each stage of the EU HTA. Due to the limited time available from PICO finalisation to the JCA submission deadline, the efficiency of decision-making and material development will be emphasised here. However, it is equally essential that the processes for JCA preparations begin early, approximately nine months to a year, before the letter of intent is sent to start the JCA and before the EMA marketing authorisation submission process begins. Developing these internal strategies will also mean defining those responsible for each of the tasks to be completed before and during the JCA and determining the timelines for completion.
Two things must be decided upon early in the preparation stage to gain cross-departmental agreement. First is alignment on the intended label for the product in question, as this must be ready for the EMA submission but will also be imperative for PICO selection and, therefore, has a significant impact on the rest of the JCA. The effect of a last-minute label change is, thus, greatly amplified compared to the current system. The final label will not be published until day 277 of the EMA/JCA process (fig 2). However, the broader implications of a change at this point are unknown. Therefore, to avoid uncertainty and ensure a smooth completion of the JCA and EMA processes in parallel, manufacturers must decide on their intended use early and may also have to accept a more conservative approach. The PICOs themselves should also be agreed upon before the JCA begins. This means that companies should put together a well-defined process for selecting PICOs and ensure they align with the label and overall submission strategy.
Secondly, early agreement on the submission’s goals, either gaining the highest possible price, a broader patient population or fast access, will be part of a larger discussion throughout the preparation phase on the strategy for the JCA value story, what the evidence needs, and a mitigation strategy if the base case needs to be adapted.
The success of these internal structures or processes will be borne out in the avoidance of duplication by global and affiliate teams, prompt development of materials (i.e., the JCA dossier being completed before the process starts), a clearly defined processes/”playbook” for JCA and the flexibility to adapt to changes in PICOs, the label and further evidence readouts.
Ensuring adequate resources available for EU HTA
As an entirely new process done in parallel with EMA submissions and, as opposed to the common misconception, done in addition to local country HTAs, this will require significant resources. Therefore, companies must act now to ensure they have the correct personnel in the right positions to succeed in JCA. It is especially urgent if products are expected to be involved in the first phase of JCAs in 2025 (new oncology medicines and ATMPs).2,3
To do this, manufacturers should be thinking about these four questions:
- Who is accountable?
Specifically, who will lead the submission at a senior level and make the final decisions about strategy and dossier content? This could be medical affairs, market access, regulatory, etc.; however, we recommend that this sits within whichever department has the most experience directing the strategy for local country submissions and is likely to be market access. Whoever is accountable for the JCA submission must collaborate closely with the regulatory body, as the EMA application is being processed simultaneously. Additionally, this speaks against regulatory accountability for the JCA as this could put too much strain on one company area and may restrict the focus to regulatory approval.
- Who is responsible?
Who will be responsible for preparing the dossier itself? The first decision to be made here is if this will be done in-house or if it will be outsourced. This decision will likely depend on the company’s resources available to each manufacturer and the available resources at the time of submission. Additionally, whether the global team will do this, regional European teams, or at the country affiliate level will need to be decided. We recommend that this takes place at the European level because they have greater regional visibility to the affiliate teams but a more in-depth understanding of the market than a global team might have. Again, this will be a decision that each manufacturer must consider themselves and depends on whether there is a regional level in place. However, the responsibility for dossier preparation must remain with the same team so that first-hand experience in putting together these submissions can be built. Additionally, the function responsible for submission preparation must also be considered (market access, regulatory, HEOR, etc) and is likely to use the same criteria as deciding which is to be accountable.
- Who has the capability?
Do the manufacturer functions have the right capabilities to undergo JCA? For example, there is likely to be more than one set of PICOs selected, and it is unlikely that all of these can be addressed just from the clinical trial. Therefore, some form of indirect treatment analysis is likely to be needed. Do companies/departments have the specialist knowledge required to address some of these? If there is no in-house capability, and the task will be outsourced, this should be addressed immediately, especially if potential products are going through the JCA in 2025.
- Who has availability?
Will additional resources be granted, or will this be done with the current resources available in-house? Companies must begin thinking about the processes to ensure the appropriate resources are given to develop all the relevant materials. Consideration also needs to be given to the very short timelines and the fact that this is a new process where prior experience is lacking. Therefore, the efficiency of the teams working on this may not be the same as that of a more established HTA submission.
Manufacturers should also plan for a worst-case scenario: If the available in-house resources are not sufficient, will this be to outsource the development of materials, and if so, where will you go for the relevant expertise?
Embed networks of impactful collaboration and communication across functions
The collaboration across functions, although essential in the past, will be critical to completing the JCA process. Previously, different organisational functions were involved in sequential effort, with limited contact points between market access, regulatory, etc. EU regulations would begin developing materials for marketing authorisation. Global/EU market access would take this on and start developing HTA evidence before handing it off to the affiliates to build it into their local submission dossiers. However, the advent of EU HTA will mandate market access collaboration and knowledge sharing earlier in development, where simultaneous effort from all the key stakeholders will be required to make the JCA dossier as robust as possible. This will involve a two-way conversation, particularly between global/EU market access and the affiliates, where local teams can provide input on what the PICOs are likely to be, what country HTA agencies are likely to think of the indirect treatment analysis, etc. It is also essential for the local affiliates to have visibility of what is going on in the JCA submission so the country’s HTA dossiers do not contain repeated information and only provide additional evidence relevant to their market.
To begin preparation for this now, companies should determine the best forms of communication across a large team, including multiple functions and levels, to facilitate effective collaboration. The outputs of this will include the establishment of dedicated management tools, such as Teams channels and SharePoint systems, to allow easy communication, document storage and virtual touchpoints throughout the submission process.
Conclusion
Given that we are just under five months away from the implementation of EU HTA, if any manufacturer has a product involved in the first phase (new oncology medicines and ATMPs), they should be ready to implement any new organisational changes immediately if they have not already. This should not be limited to manufacturers involved in the first stage. Any company whose products are due to enter the EU HTA in the second phase should also begin the same preparation.
It must be emphasised that not “one size fits all” for the specific way resources will be allocated for JCA submissions. However, the preparations made by manufacturers should be guided by the principles outlined in this article. Where the three key considerations should be:
- Who does what and when – Clarity within the organisation on who leads EU HTA dossier submissions
- Are current resources adequate – EU HTA occurs parallel to EMA evaluation and is initially likely to increase resource requirements
- How to collaborate effectively
For further information on how to start planning and implementing changes immediately in preparation for EU HTA, watch our webinar on taking action. For more tailored help, get in touch to see how Remap can help develop materials for JSC and JCA or with bespoke training.5,6
Key actions companies should be thinking about
- Start restructuring your organisation early to ensure you have the right resources in place to facilitate JCA dossier development
- Estimate the likely resources needed for JCA and where they are going to come from
- Initiate internal education on the importance of collaborative market access and the need to get the EU HTA right
Sources:
1. European Commission. Implementation of the Regulation on Health Technology Assessment. Accessed 06/08/2024, https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment/implementation-regulation-health-technology-assessment_en
2. The European Parliament and the Council of the European Union. REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU Official Journal of the European Union2021.
3. European Union. Factsheet on the Regulation: Implementation of the EU Health Technology Assessment Regulation. Accessed 12/08/2024, https://health.ec.europa.eu/system/files/2023-09/hta_regulation-implementation_factsheet_en.pdf
4. Brodie M. Ask not why but how, says UK collaboration architect. Accessed 19/08/2024, https://mednews.com.au/why-collaborate-is-the-question-architect/
5. Remap Consulting. EU HTA: Moving from strategy to action. Accessed 15/08/2024, https://remapconsulting.com/hta/eu-hta-moving-from-strategy-to-action/
6. Remap Consulting. Implications of the EU HTA process for Manufacturers. Accessed 15/08/2024, https://remapconsulting.com/hta/implications-of-the-eu-hta-process-for-manufacturers/